Abstract
Introduction
Hypoactive sexual desire disorder (HSDD) often has a negative impact on the health and quality of life of women; however, many women do not mention—let alone discuss—this issue with their physicians. Providers of gynecologic services have the opportunity to address this subject with their patients.
Aim
To review the diagnosis and evidence-based treatment of low sexual desire in women with a focus on strategies that can be used efficiently and effectively in the clinic.
Methods
The Medline database was searched for clinically relevant publications on the diagnosis and management of HSDD.
Results
HSDD screening can be accomplished during an office visit with a few brief questions to determine whether further evaluation is warranted. Because women’s sexual desire encompasses biological, psychological, social, and contextual components, a biopsychosocial approach to evaluating and treating patients with HSDD is recommended. Although individualized treatment plan development for patients requires independent medical judgment, a simple algorithm can assist in the screening, diagnosis, and management of HSDD. Once a diagnosis of HSDD has been made, interventions can begin with office-based counseling and progress to psychotherapy and/or pharmacotherapy. Flibanserin, a postsynaptic 5-hydroxytryptamine 1A agonist and 2A antagonist that decreases serotonin levels and increases dopamine and norepinephrine levels, is indicated for acquired, generalized HSDD in premenopausal women and is the only agent approved in the United States for the treatment of HSDD in women. Other strategies to treat HSDD include using medications indicated for other conditions (eg, transdermal testosterone, bupropion). Bremelanotide, a melanocortin receptor agonist, is in late-stage clinical development.
Conclusions
Providers of gynecologic care are uniquely positioned to screen, counsel, and refer patients with HSDD. Options for pharmacotherapy of HSDD are currently limited to flibanserin, approved by the US Food and Drug Administration, and off-label use of other agents.
Clayton AH, Kingsberg SA, Goldstein I. Evaluation and Management of Hypoactive Sexual Desire Disorder. Sex Med 2018;6:59–74.
Key Words: Sexual Dysfunction, Hypoactive Sexual Desire Disorder, Flibanserin, Screening, Diagnosis, Drug Therapies
Introduction
Hypoactive sexual desire disorder (HSDD) is defined as a persistent or recurrent deficiency (or absence) of sexual fantasies and desire for sexual activity that causes marked distress or interpersonal difficulty not related to a medical or psychiatric condition or the use of a substance or medication.1, 2 The clinician also should take into account the context of the person’s life (eg, severe relationship problems) when evaluating loss or absence of sexual desire.1, 3 HSDD is common in women but is often unaddressed or undertreated.4 Large population-based studies have shown that approximately 36% to 39% of women report low sexual desire, with 8% to 10% meeting the primary diagnostic criteria for HSDD (low desire and associated distress).5, 6 Similarly, the prevalence of HSDD equaled 7.4% in a cohort of women who received routine medical care from 20 obstetrics and gynecology or primary care clinics.7 In women, low sexual desire generally increases with age, whereas related distress decreases, resulting in a fairly steady prevalence of HSDD across the adult lifespan.8 Consistent with this observation, a questionnaire-based cross-sectional study of 1,548 Australian women 65 to 79 years of age reported a prevalence of low sexual desire, sexually related personal distress, and HSDD of 88.0%, 15.5%, and 13.6%, respectively.9
Low sexual desire can have a substantial negative impact on women’s health and quality of life.10, 11, 12, 13 However, surveys of women who self-reported low sexual desire and associated distress showed that up to 80% had not mentioned the issue to a health care provider, with at least 50% reporting that discomfort or embarrassment contributed to their unwillingness to seek treatment.13, 14 Providers who deliver gynecologic services are uniquely positioned to identify and address patients’ concerns about sexual functioning. This discussion of the literature on diagnosis and evidence-based treatment of low sexual desire in women focuses on strategies that can be used efficiently and effectively in the clinic.
Methods
The Medline database was searched using the terms (“screening” OR “diagnosis” OR “management” OR “treatment”) AND (“hypoactive sexual desire disorder” OR “HSDD”). For this narrative review, selection of publications relevant to the clinical practice of gynecologic service providers was based on the authors’ clinical and research expertise.
Pathophysiology
Distressing low sexual desire can be attributed to a number of biological, psychological, social, and contextual components.15, 16, 17 Although biomedical factors—such as altered hormones and neurotransmitters and their interactions, genetics, and medical and psychiatric conditions—can explain aspects of HSDD, it is important to understand the complexity of the female sexual response and how other factors can contribute to HSDD. These include psychological factors, such as boredom, situational stress, self-consciousness about body image, and distraction; and social and contextual factors that include cultural norms, familial teachings, and relationship considerations.
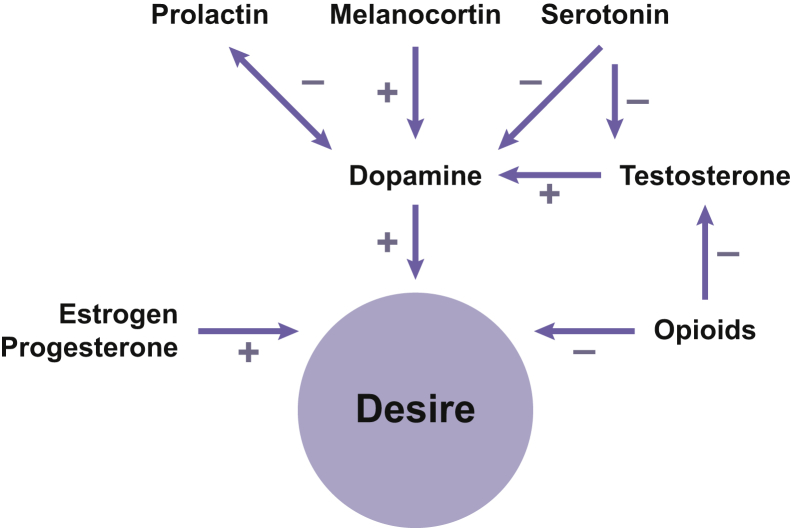
The neurochemical basis of HSDD has not been fully elucidated; however, it is currently understood that low sexual desire results from hypofunctional excitation, hyperfunctional inhibition, or a combination of the 2.18, 19 Sexual desire is believed to be regulated by neuromodulators (neurotransmitters and hormones) of excitatory pathways (eg, dopamine, norepinephrine, melanocortins, oxytocin) and inhibitory pathways (eg, serotonin, opioids, endocannabinoids; Figure 1).17, 18, 19 Decreased neural activation of brain regions associated with sexual arousal (eg, medial orbitofrontal region and periaqueductal gray matter) and a lack of disinhibition of brain regions involved in cognitive processing (eg, left brain) in women with HSDD can impair vaginal vasocongestion and lubrication and perhaps decrease female orgasm.20
Figure 1.
Excitatory and inhibitory effects of neurotransmitters and hormones on sexual desire.
Figure adapted with permission from John Wiley & Sons, Inc, from Clayton AH. Int J Gynaecol Obstet 2010;110:7–11,17 ©2010 International Federation of Gynecology and Obstetrics. Published by Elsevier Ireland Ltd. All rights reserved.
Recognition and diagnosis of HSDD
The central features of HSDD are low sexual desire and associated distress. The International Classification of Disease (ICD-10 and its anticipated update, ICD-11) include a diagnostic code for HSDD (F52.0 in ICD-10) defining HSDD as an “absence or marked reduction in desire or motivation to engage in sexual activity as manifested by any of the following: 1) reduced or absent spontaneous desire (sexual thoughts or fantasies); 2) reduced or absent responsive desire to erotic cues and stimulation; or 3) inability to sustain desire or interest during sexual activity. The pattern is persistent or recurrent over a period of at least several months and occurs frequently, though may fluctuate in severity, and is not secondary to a sexual pain disorder. The symptoms are associated with clinically significant distress.”21 The International Society for the Study of Women’s Sexual Health has a similar broad definition but also notes that HSDD is not situational in nature (ie, not secondary to physical and/or emotional abuse, dissatisfaction with the partner, or intrusion of life stressors that can be affected by psychological and/or lifestyle changes).3, 22 Before diagnosing HSDD, relationship issues (eg, significant relationship conflict) should be ruled out as a primary causative factor.3
Although the ICD system continues to include a diagnostic code for HSDD, the most recent update to the American Psychiatric Association diagnostic manual (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) merged HSDD and female sexual arousal disorder to create a new diagnostic category, “female sexual interest and arousal disorder” (FSIAD).23 The new FSIAD diagnosis is controversial among experts in sexual medicine, in large part because of the lack of information on validity and clinical utility.24 Different symptom patterns have been observed in women with HSDD and FSIAD, and using the new criteria could exclude from diagnosis (and therefore treatment) a large number of women with HSDD of moderate to marked, rather than severe, intensity.25 Because research and literature on FSIAD are lacking,24 the data reviewed in this article on the etiology and management of low sexual desire in women are based on the previous diagnostic criteria (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision).
Barriers to Diagnosis
Although the symptoms of HSDD have been clearly defined,1 recognizing low sexual desire or distressing sexual problems is complicated by the fact that women often do not spontaneously mention these issues to their health care providers. In a survey of 450 premenopausal and postmenopausal women with self-reported low sexual desire, results showed that 73% of premenopausal women and 81% of postmenopausal women reported having never mentioned their desire problems to a health care provider.13 In a larger survey, only 34.5% of 3,239 women with a distressing sexual problem had formally discussed their problems with their health care providers.14 In the 2 surveys, embarrassment contributed to women’s unwillingness to seek help from a health care provider.13, 14
Because of this, it is incumbent on providers of gynecologic services to broach the subject with their patients. However, many fail to initiate a discussion about patients’ sexual health,26 citing reasons that include limited time with patients, discomfort with the topic, and a perceived lack of effective therapies.27, 28 Results from a self-administered survey completed by 1,196 women who sought routine Pap smear screening at a family practice clinic or an obstetrics and gynecology clinic showed that, for at least 45% of patients, the topic of sexual health was not raised during their visit.26 However, most of these women (∼85%) expressed that the topic would be easier to discuss if the provider broached the topic, which indicates an untapped opportunity for improved communication and care.
Engaging the Patient: Screening for HSDD
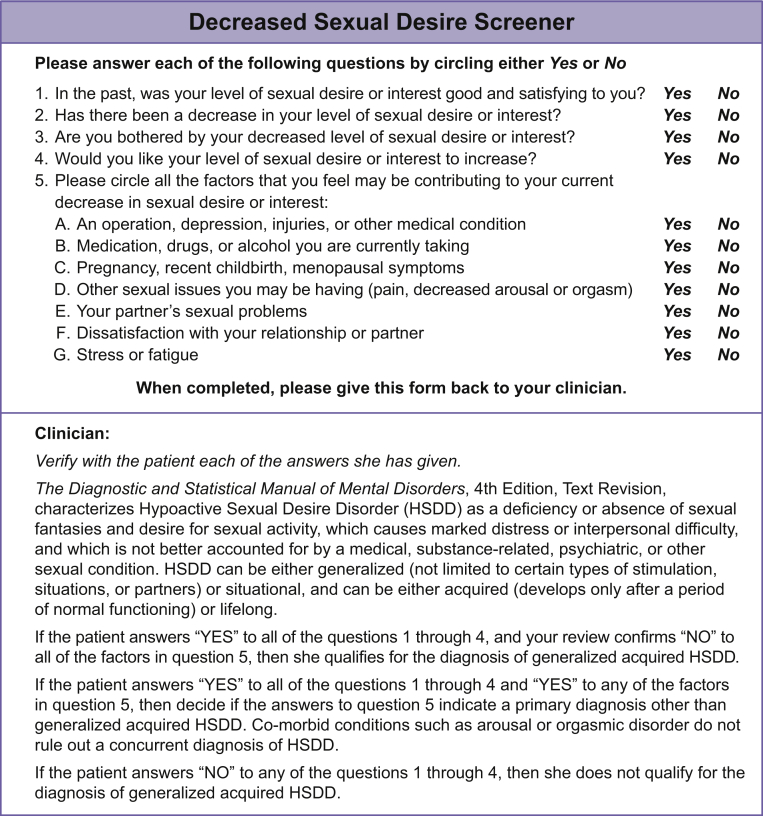
Screening for HSDD can be accomplished in a time-efficient manner during an office visit. A few brief questions could determine whether further evaluation is warranted. For example, are you sexually active? If not, why not? What sexual concerns do you have? How do you feel about your level of sexual desire, arousal, or orgasm? To facilitate these discussions, ensure a safe, non-judgmental environment for the patient.29 This could include educating and training staff to be comfortable with sexual topics, offering patient-friendly materials in the waiting and examination rooms, and including questions about sexual topics on intake forms. To assist in the diagnosis of acquired, generalized HSDD, the Decreased Sexual Desire Screener is a 5-item validated questionnaire designed for clinical practice (Figure 2)30 and is available online.31 Although screening for HSDD is easily performed as part of an office visit, follow-up might be necessary to address sexual concerns and to provide office-based counseling or medication management.29
Figure 2.
Decreased Sexual Desire Screener.
Figure adapted with permission of the copyright holder, Boehringer Ingelheim International GmbH, © 2005. All rights reserved.
Diagnosis of HSDD
If screening suggests the presence of HSDD, the next steps should include soliciting more detailed information from patients about their experience of low desire, including onset, duration, behavioral adaptation and avoidance, and level of distress. Close evaluation of the patient’s medical history (including reproductive history), comorbid conditions (including endocrine, neurologic, cardiovascular, and psychiatric disorders), use of prescription and over-the-counter medications (Table 1), and a targeted physical examination (as indicated) also should take place to rule out any other causes of low desire.16, 29, 32 Psychiatric disorders, in particular depression and anxiety, also should be considered in the differential diagnosis as potential contributing factors to low desire.29 Oral contraceptives have been associated with low desire in some studies but not in others, so the potential role of these agents also should be assessed.29 Laboratory tests are usually of limited usefulness in establishing a diagnosis of HSDD, although specific tests might be warranted based on patient history and physical examination, such as physical findings suggestive of thyroid disease or hyperprolactinemia.29
Table 1.
Medications associated with low sexual desire∗
| Drug class | Medications |
|---|---|
| Antiepileptic drugs | Carbamazepine, phenytoin, primidone |
| Cardiovascular and antihypertensive agents | ACE inhibitors, amiodarone, β-blockers (atenolol, metoprolol, propranolol), calcium channel blockers, clonidine, digoxin, diuretics (hydrochlorothiazide, spironolactone), lipid-lowering agents |
| Hormonal medications | Antiandrogens (flutamide), GnRH agonists, oral contraceptive pills |
| Pain relievers | NSAIDs, opiates |
| Psychotropic medications | Prolactin-inducing antipsychotics, anxiolytics (alprazolam, diazepam), lithium, SNRIs, SSRIs, tricyclic antidepressants |
| Other | Chemotherapeutic agents, histamine receptor blockers, indomethacin, ketoconazole |
| Drugs of abuse | Alcohol, amphetamines, cocaine, heroin, marijuana |
ACE = angiotensin-converting enzyme; GnRH = gonadotropin-releasing hormone; NSAID = non-steroidal anti-inflammatory drug; SNRI = serotonin-norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor.
Treatment
Because sexual desire encompasses biological, psychological, social, and contextual components, a biopsychosocial approach to the treatment of women with HSDD is warranted.15, 16, 17 An initial approach might be office-based counseling to provide basic education on the diagnosis and correct misperceptions about human sexuality and recommendations for behavioral or lifestyle changes to improve self-esteem and increase sexual interest and intimacy.29 This approach could be helpful until patients make the decision to engage in psychotherapy and/or pharmacotherapy.16 The PLISSIT (Permission, Limited Information, Specific Suggestions, Intensive Therapy) model, an incremental approach to office-based counseling, could be a useful approach for practitioners.33 With the PLISSIT model, women are given permission (P) to discuss their problems and emotions and to explore solutions. Then, the practitioner provides limited information (LI), which includes basic sexual function education and/or resources (eg, literature, videos, and erotica), and specific suggestions (SS) for addressing the problem in the form of directives and advice. If the individual needs more intensive treatment (IT) for HSDD, then the practitioner can refer her for individual or couple’s therapy.
Psychological Intervention
Referral to a psychotherapist is often the next step once office-based counseling proves inadequate or if, during counseling, the presence of trauma, abuse, or serious psychiatric issues comes to light.29 Psychotherapy focuses primarily on the psychological and sociocultural factors contributing to distressing low desire. Interventions for female sexual dysfunction can include cognitive-behavior therapy,34, 35, 36 mindfulness training,37, 38 and couples’ exercises including sensate focus,39 bibliotherapy,40, 41, 42 and use of erotic materials (if acceptable to the patient).43 Although such interventions are reportedly beneficial for patients with HSDD, empirical evidence from controlled trials is limited.44, 45
Evidence-Based Pharmacologic Treatment Options
In randomized controlled clinical trials of HSDD, outcomes were assessed using validated patient-reported outcomes. The Female Sexual Function Index (FSFI) is a validated 19-item questionnaire that assesses the main dimensions of sexual function: desire, arousal, lubrication, orgasm, global satisfaction, and pain.46 The desire domain (FSFI-d) assesses the frequency and degree of desire. The Female Sexual Distress Scale–Revised (FSDS-R) is a questionnaire that allows the measurement of distress related to sexual dysfunction and specifically low desire using item 13 (FSDS-R-13).47 Although satisfying sexual events (SSEs) were counted as a co-primary end point in some studies, a lack of SSEs is not a criterion for the diagnosis of HSDD. Rather, SSEs seem to be a downstream measure of improvements in sexual desire and associated distress.
Flibanserin
Flibanserin (Valeant Pharmaceuticals North America LLC, Bridgewater, NJ, USA), the only agent approved by the US Food and Drug Administration (FDA) for the treatment of HSDD,48 is indicated for acquired, generalized HSDD in premenopausal women and is administered as a 100-mg tablet once daily at bedtime.49, 50 This agent (postsynaptic 5-hydroxytryptamine 1A agonist and 2A antagonist) decreases serotonin levels and increases dopamine and norepinephrine levels, which are neurotransmitters that affect sexual desire.18, 51 Flibanserin is believed to work on brain function by enhancing excitatory elements and lessening the inhibitory response to sexual cues.52
Flibanserin has been evaluated in a number of clinical trials in the treatment of patients with HSDD (Table 2).53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 The approval of flibanserin by the FDA was based on results from 3 randomized placebo-controlled trials of premenopausal women with HSDD that showed statistically significant improvement for flibanserin 100 mg (daily at bedtime) vs placebo in sexual desire (per the FSFI-d score), desire-related distress (per the FSDS-R-13 score), and the number of SSEs.53, 54, 55 The most common adverse events in premenopausal women treated with flibanserin 100 mg at bedtime (incidence >2% and more often than placebo-treated patients) were dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.49
Table 2.
Studies of flibanserin in the clinical development program for HSDD
| Study | Type of study | Patients and study medication | Key findings |
|---|---|---|---|
| Phase 3 studies | |||
| VIOLET53 | R, DB, PC; 24 wk | Premenopausal women with HSDD (n = 295 placebo, n = 295 flibanserin 50 mg qhs, n = 290 flibanserin 100 mg qhs) | Significant improvement in FSFI desire score, FSFI total score, FSDS-R-13 score, and number of SSEs for flibanserin 100 mg/d vs placebo; most common AEs with flibanserin 100 mg (≥5% and 2 × placebo): nausea (11.4%), somnolence (11.0%), dizziness (9.0%), fatigue (6.2%) |
| DAISY54 | R, DB, PC; 24 wk | Premenopausal women with HSDD (n = 398 placebo, n = 396 flibanserin 25 mg bid, n = 392 flibanserin 50 mg bid, n = 395 flibanserin 100 mg qhs) | Significant improvement in FSFI desire score, FSFI total score, FSDS-R-13 score, and number of SSEs for flibanserin 100 mg/d vs placebo; most common AEs with flibanserin 100 mg (≥5% and 2 × placebo): dizziness (12.2%), nausea (11.9%), somnolence (11.9%); discontinuation due to AEs: 15.7% of patients treated with flibanserin 100 mg |
| BEGONIA55 | R, DB, PC; 24 wk | Premenopausal women with HSDD (n = 545 placebo, n = 542 flibanserin 100 mg qhs) | Significant improvement in FSFI desire score, FSFI total score, FSDS-R-13 score, and number of SSEs for flibanserin 100 mg/d vs placebo; most common AEs with flibanserin 100 mg (≥5% and 2 × placebo): somnolence (14.4%), dizziness (10.3%), nausea (7.6%), URI (5.2%); discontinuation due to AEs: 9.6% of flibanserin-treated patients |
| SNOWDROP56 | R, DB, PC; 24 wk | Naturally postmenopausal women with HSDD (n = 480 placebo, n = 467 flibanserin 100 mg qhs) | Significant improvement in FSFI desire score, FSFI total score, FSDS-R-13 score, and number of SSEs for flibanserin 100 mg/d vs placebo; most common AEs with flibanserin 100 mg (≥5% and 2 × placebo): dizziness (9.9%), somnolence (8.8%), nausea (7.5%); discontinuation due to AEs: 8.1% of flibanserin-treated patients |
| PLUMERIA57 | R, DB, PC | Naturally postmenopausal women with HSDD (n = 369 placebo, n = 376 flibanserin 100 mg qhs) | Study discontinued for administrative reasons∗; week 16 used as study end point; most common AEs with flibanserin 100 mg (≥5% in either treatment group): insomnia (7.7%), somnolence (6.9%); discontinuation due to AEs: 10.4% of flibanserin-treated patients; at week 16, significant improvement in FSFI desire score for flibanserin vs placebo; between-group comparison for SSE did not reach statistical significance |
| ROSE58 | R, DB, PC, withdrawal study | Premenopausal women with HSDD (n = 738 received OL flibanserin, flexibly dosed); treatment responders at OL week 24 were randomly assigned (n = 170 placebo, n = 163 flibanserin) | Sustained efficacy with continued flibanserin (ie, significantly better outcomes with flibanserin vs placebo in FSFI desire score, FSFI total score, FSDS-R-13 score, and number of SSEs at DB end point); most common AEs with OL flibanserin (≥10%): somnolence (14.1%), fatigue (10.3%); discontinuation due to AEs: 2.5% of flibanserin-treated patients in DB phase and 15.2% of flibanserin-treated patients in OL phase; no withdrawal reactions reported after abrupt discontinuation of flibanserin |
| SUNFLOWER59 | OL extension study; 52 wk | Premenopausal women with HSDD (n = 1723 OL flibanserin, flexibly dosed) | Incidence of prespecified AEs of interest: somnolence (15.8%), fatigue (7.6%), dizziness (6.9%), nausea (6.3%), sedation (1.6%), vomiting (1.4%); discontinuation due to AEs: 10.7% of patients; serious AEs reported in 1.2% of patients; none considered drug-related by the investigator; improvement in measurements of sexual desire (FSFI desire score, FSFI total score, FSDS R-13 score) |
| SSRI/SNRI study60 | R, DB, PC; 12 wk; study designed and powered to assess safety | Premenopausal women with remitted or mild depressive disorder and decreased sexual desire with related distress taking SSRI or SNRI (n = 38 placebo, n = 73 flibanserin 100 mg qhs [including n = 45 up-titrated after 2 wk of 50 mg qhs]) | Study discontinued for administrative reasons∗; most common AEs with flibanserin (≥3% and > placebo): dry mouth (5.5%), insomnia (5.5%), back pain (4.1%), dizziness (4.1%); no increased risk of depression, anxiety, or suicidal ideation when flibanserin added to stable SSRI or SNRI treatment regimen; no significant differences between flibanserin and placebo on exploratory outcome measures |
| Other studies | |||
| Alcohol challenge study61 | R, DB, PC single-dose, crossover study | Healthy volunteers (n = 23 men, n = 2 women); flibanserin 100 mg or placebo with ethanol 0.4 or 0.8 g/kg or flibanserin alone, consumed within 10 min in the morning | Incidence of sedation-, hypotension-, and syncope-related AEs was increased when flibanserin was coadministered with either concentration of alcohol |
| Alcohol use analysis61 | Pooled post hoc analysis of 5 RCTs | Premenopausal women with HSDD (n = 1,905 placebo [64% self-reported alcohol users], n = 1,543 flibanserin 100 mg qhs [58% self-reported alcohol users]) | Rate of hypotension- and syncope-related AEs during treatment with flibanserin was slightly higher numerically in self-reported alcohol users vs non-users, although the incidence of such events was infrequent (0.7% vs 0.3%); rate of hypotension- and syncope-related AEs was similar (0.3%) for alcohol users vs non-users in placebo group |
| Driving study62 | R, DB, PC, crossover study | Healthy premenopausal women (n = 72 completed all 4 treatment periods); zopiclone 7.5 mg qhs on nights 1 and 7 with 5 d of placebo in between; flibanserin 100 mg qhs × 7 d; flibanserin 200 mg qhs (2 × approved dose) on night 7 after 6 nights of 100 mg; placebo × 7 d | Flibanserin 100 mg was significantly better than placebo in driving simulation and cognitive assessments after acute and steady-state night-time dosing; no significant difference for flibanserin 100 mg vs 200 mg; impairment with zopiclone (sedative) vs placebo supported sensitivity of outcome measures |
| Oral contraceptive PK study63 | Meta-analysis of 9 phase 1 studies (effect of oral HC use on flibanserin PK) | Healthy female volunteers and patients with HSDD; flibanserin (25 mg qd, 50 mg qd, 50 mg bid, 100 mg qd); single-dose flibanserin (n = 39 [HC users], n = 114 [HC non-users]) and multiple-dose (ie, steady-state) flibanserin (n = 17 [HC users], n = 91 [HC non-users]) | AUC0-∞ 1.42-fold higher (90% CI = 1.23–1.63) after single-dose flibanserin; AUC0-T 1.44-fold higher (90% CI = 1.19–1.73) at steady state in HC users vs non-users |
| Oral contraceptive PK study64 | R, OL, crossover study (effect of flibanserin on oral contraceptive PK) | Healthy premenopausal women (N = 24 [23 completed both treatment periods]); single doses of ethinyl estradiol 30 μg + levonorgestrel 150 μg alone (reference) or preceded by 14 d of flibanserin 100 mg qhs (test) | Pretreatment of flibanserin 100 mg once daily for 2 wk produced no clinically relevant change in single-dose PK of ethinyl estradiol or levonorgestrel; incidence of AEs was higher for flibanserin before oral contraceptive administration vs oral contraceptive alone but did not increase after coadministration |
| Population PK study65 | Single-dose PK study | Healthy volunteers (n = 14 men, n = 10 women); 1 dose of flibanserin 100 mg after overnight fast | In PK/PD model, rapid drug distribution was identified; relation of drug concentration with drowsiness (assessed by VAS) indicated minimal sedating effect of flibanserin at concentrations typically observed 4 h after administration |
AE = adverse event; AUC0-∞ = area under the dose-normalized concentration-time curve extrapolated to infinity; AUC0-T = area under the dose-normalized concentration-time curve over the dosing interval; bid = twice daily; DB = double-blinded; FSFI = Female Sexual Function Index; FSDS-R-13 = Female Sexual Distress Scale–Revised, Item 13; HC = hormonal contraceptive; HSDD = hypoactive sexual desire disorder; OL = open-label; PC = placebo-controlled; PD = pharmacodynamic(s); PK = pharmacokinetic(s); qd = daily; qhs = at bedtime; R = randomized; RCT = randomized controlled trial; SNRI = serotonin-norepinephrine reuptake inhibitor; SSE = satisfying sexual events; SSRI = selective serotonin reuptake inhibitor; URI = upper respiratory tract infection; VAS = visual analog scale.
The development of flibanserin in this population was discontinued by the sponsor (Boehringer Ingelheim).
In clinical trials of flibanserin, some of the most common adverse events included somnolence and dizziness, which raised safety concerns during FDA review with regard to alcohol consumption.
To address these concerns, an alcohol challenge study with 25 healthy adult male (n = 23) and female (n = 2) volunteers was conducted.61 In the morning, in 10 minutes and on a nearly empty stomach, volunteers consumed 95% ethanol alcohol 0.4 g/kg (a weight-based approximation of 2 shots of distilled spirits or 2 12-oz cans of beer) or 0.8 g/kg (a weight-based approximation of 4 shots of distilled spirits or 4 12-oz cans of beer) mixed in orange juice, or orange juice alone, along with flibanserin 100 mg or placebo. The incidence of sedation-, hypotension-, and syncope-related adverse events was increased when flibanserin was coadministered with either concentration of alcohol. However, in a post hoc analysis of 5 randomized, placebo-controlled, 6-month flibanserin trials, the incidence of hypotension- and syncope-related adverse events in flibanserin-treated patients was low for self-reported alcohol users (0.7%) and alcohol non-users (0.3%).61
Because of the concern of hypotension and syncope associated with a flibanserin and alcohol interaction, the flibanserin package insert indicates that alcohol use is contraindicated in women taking flibanserin,49 requiring a Risk Evaluation and Mitigation Strategy (REMS) for the drug. The REMS program (available at https://www.addyirems.com/AddyiUI/rems/home.action) delineates the steps that must be taken by prescribers and pharmacists for patients to receive flibanserin. As a requirement of the REMS, health care providers who wish to prescribe flibanserin must review the drug’s prescribing information, review a Prescriber and Pharmacy Training Program, complete a Knowledge Assessment Form, and submit the Prescriber Enrollment Form online, with a similar certification and enrollment process for pharmacies to dispense flibanserin.66
Because flibanserin is indicated for use in premenopausal women, the ability to use it with oral contraceptives is of interest to clinicians. A study of 24 healthy premenopausal women found no clinically relevant effects of flibanserin on the pharmacokinetic profile of a combination ethinyl estradiol and levonorgestrel oral contraceptive pill, suggesting that coadministration of flibanserin might not affect the efficacy of the contraceptive.64 Pregnancy data from the flibanserin clinical development program support this finding (data on file).
Other Pharmacologic Approaches to HSDD
The search for effective agents to manage HSDD has been pursued for decades; medications indicated for other conditions (ie, testosterone, bupropion) have been used off-label in the treatment of HSDD (Table 3).67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 Testosterone is the primary sex hormone involved in the regulation of sexual desire.17 Although high-quality studies in premenopausal women are lacking,78 evidence from multiple phase 2 and 3 studies supports the efficacy of transdermal testosterone, which increases androgen levels, for improving desire and sexual satisfaction in postmenopausal women with HSDD.68, 69, 70, 71, 72, 73, 74, 79, 80, 81, 82, 83 A recent systematic review and meta-analysis included 7 randomized controlled studies of transdermal testosterone (with or without concomitant estrogen therapy) that was composed of more than 3,000 postmenopausal women with HSDD.67 Postmenopausal women treated with the transdermal testosterone patch experienced significant increases in sexual desire, sexual activity, SSE, and orgasms and a significant decrease in personal distress compared with women in the placebo group.67 However, the FDA did not approve a testosterone transdermal patch (Proctor & Gamble, Cincinnati, OH, USA; Watson Pharmaceuticals, Corona, CA, USA) intended to treat HSDD in postmenopausal women.84 Although improvements in primary (SSE) and secondary (sexual desire, distress) efficacy outcomes were significantly greater for the testosterone patch compared with placebo in phase 3 studies of surgically menopausal women,69, 70 the FDA advisory committee voted that long-term safety data were inadequate to support approval,85 although the testosterone patch was approved in the European Union for use in this population. A testosterone gel (BioSante Pharmaceuticals, Lincolnshire, IL, USA) evaluated in surgically menopausal women with HSDD failed to meet primary and secondary end points in phase 3 studies.75 The availability of transdermal testosterone for women is limited to unregulated formulations and off-label use of products intended for men, which can lead to higher than normal androgen levels and androgen-related side effects.80
Table 3.
Agents used off-label for the treatment of HSDD
| Agent | Study | Study design | Key findings |
|---|---|---|---|
| Transdermal testosterone | Achilli et al, 201767 | Meta-analysis of 7 RCTs68, 69, 70, 71, 72, 73, 74; postmenopausal women (N = 3,035); testosterone patch vs placebo or no-treatment control | Significantly greater improvement in number of SSEs, sexual desire, and personal distress with testosterone vs placebo; no significant difference between testosterone patch and placebo in overall incidence of AEs, severe AEs, or study discontinuation; significantly more androgenic AEs (ie, acne, hair growth) for testosterone vs placebo |
| Waldman et al, 201275; NCT00613002, NCT00657501 | 2 R, DB, PC studies; surgically menopausal women with HSDD (N = 1,172); testosterone gel (300 μg/d) | No significant differences between testosterone gel and placebo in sexual desire or number of SSEs | |
| Bupropion | Segraves et al, 200476 | R, DB, PC study; 16 wk; premenopausal women with HSDD (n = 31 bupropion SR, n = 35 placebo); bupropion SR 300 mg/d with optional increase to 400 mg/d | Significant increase for bupropion SR vs placebo on some measurements of sexual function (CSFQ total, arousal, and orgasm scores) but not others (CSFQ desire score, Brief Index of Sexual Functioning in Women) |
| Segraves et al, 200177 | 4-wk single-blinded baseline phase followed by 8-wk single-blinded treatment phase; premenopausal and postmenopausal women with HSDD (N = 51); bupropion SR 150 mg bid | 29% of patients were treatment responders (“much improved” or “very much improved” on CGI-I); most common AEs with bupropion (≥5% of patients and > placebo) were insomnia, tremor, and rash |
AE = adverse event; bid = twice daily; CGI-I = Clinical Global Impression–Improvement; CSFQ = Changes in Sexual Functioning Questionnaire; DB = double-blinded; HSDD = hypoactive sexual desire disorder; PC = placebo-controlled; R = randomized; RCT = randomized controlled trial; SR = sustained release; SSE = satisfying sexual event.
Decrease in estrogen levels, as typically occurs during the menopausal transition, has been associated with vulvovaginal atrophy and sexual dysfunction.86 A systematic review and meta-analysis of randomized controlled trials found a positive association between hormone therapy (estrogen alone or in combination with progestogens) and improvement in sexual function in women with menopausal symptoms or in early menopause.87 The beneficial effect of hormone therapy was observed primarily for pain with sexual activity; evidence specific to sexual desire is lacking.87 The recent update to the International Society of Sexual Medicine Consensus recommendations concluded that, although topical vaginal estrogen is first-line therapy for vulvovaginal atrophy, the available evidence does not support the use of systemic estrogen therapy for female sexual dysfunction (eg, HSDD).88
In studies of patients with major depressive disorder, a lower incidence of sexual dysfunction was observed for bupropion, a dopamine and norepinephrine reuptake inhibitor, compared with other agents.89, 90, 91, 92, 93 In small studies of women with HSDD, improvement in some measures of sexual function was significantly greater with bupropion sustained release compared with placebo (Table 3).76, 77 The combination of bupropion and trazodone acts to increase dopamine and norepinephrine and modulate serotonin. In a study of premenopausal women with HSDD, a proprietary combination of bupropion plus trazodone (S1 Biopharma, Inc, New York, NY, USA) was shown to be superior to the control group (bupropion alone) in the proportion of responders on standard measures of sexual desire (FSFI-d, FSDS-R-13; Table 4)94, 95, 96, 97, 98, 99, 100, 101; additional studies are planned.
Table 4.
Agents under investigation for treatment of HSDD∗
| Agent | Study | Study design | Key findings |
|---|---|---|---|
| Bremelanotide | Clayton et al, 201694 | R, DB, PC study; 12 wk; premenopausal women with HSDD (n = 92), FSAD (n = 12), or mixed (n = 290); subcutaneous bremelanotide (0.75, 1.25, or 1.75 mg) ∼45 min before anticipated sexual activity | Significant improvement in number of SSEs, FSFI total score, and FSDS-DAO score for bremelanotide (1.25 and 1.75 mg, doses pooled) vs placebo; most common AEs with bremelanotide 1.25 and 1.75 mg: nausea (22% and 24%, respectively) flushing (14% and 17%), headache (9% and 14%); discontinuation due to AEs: 5.1% and 6.1% for bremelanotide 1.25 and 1.75 mg, respectively |
| Clayton et al, 201795; Derogatis et al, 201796 | 2 R, DB, PC studies; 24 wk; premenopausal women with HSDD (N = 1,202); subcutaneous bremelanotide 1.75 mg before anticipated sexual activity | In both studies, significant improvement in FSFI-d and FSDS-DAO Desire scores (primary end points) for bremelanotide vs placebo; no significant between-treatment difference in change in number of SSEs | |
| Clayton et al, 201797 | R, DB, PC, single-dose, crossover, alcohol interaction study; healthy volunteers (n = 12 men, n = 12 women); intranasal bremelanotide 20 mg, ethanol 0.6 g/kg | No significant increase in incidence of AEs, no clinically relevant changes in blood pressure, and no PK interactions when bremelanotide was coadministered with alcohol | |
| Bupropion + trazodone | Pyke et al, 201598 | OL crossover study; 4 wk per treatment; premenopausal women with HSDD (N = 30); low- or moderate-dose bupropion + trazodone or bupropion 300 mg/d | Significantly more treatment responders with moderate-dose bupropion + trazodone vs bupropion 300 mg/d on FSFI-d (76% vs 38%) and FSDS-R-13 (88% vs 45%); most common AEs with moderate-dose bupropion + trazodone: dry mouth (53.8%), somnolence (34.6%), constipation (23.1%), insomnia (23.1%); discontinuation due to AEs: 3.8% for moderate-dose bupropion + trazodone, 0% for bupropion 300 mg/d |
| Testosterone + sildenafil† | Poels et al, 201399 | R, DB, PC, crossover study; 4 wk each of active treatment and placebo; premenopausal or postmenopausal women with HSDD (n = 24) or FSAD (n = 5) and relative insensitivity for sexual cues; sublingual testosterone 0.5 mg with sildenafil 50 mg (gelatin capsule) | Significant increases in subjective indices of sexual function (sexual desire, arousal) for testosterone + sildenafil vs placebo; most common AEs with testosterone + sildenafil: flushing (23.0%), headache (15.9%) |
| Testosterone + buspirone† | van Rooij et al, 2013100 | R, DB, PC, crossover study; 4 wk each of active treatment and placebo; premenopausal or postmenopausal women with HSDD (n = 23) or FSAD (n = 5) and over-activation of sexual inhibitory mechanisms; sublingual testosterone 0.5 mg with buspirone 10 mg (gelatin capsule) | Significant increases in subjective indices of sexual function (sexual desire, arousal) for testosterone + buspirone vs placebo; most common AEs with testosterone + buspirone: dizziness (11.3%), lightheadedness (10.3%) |
| Tribulus terrestris | de Souza et al, 2016101 | R, DB, PC study; 120 d; postmenopausal women with HSDD (N = 36); T terrestris 750 mg (3 250-mg tablets) | No significant differences between T terrestris and placebo on FSFI domain or total scores; improvement on QS-F domains of desire, arousal/lubrication, pain, and anorgasmia with T terrestris; no improvement with placebo; increase in bioavailable testosterone with T terrestris but not placebo; 3 patients in each treatment discontinued due to AE of nausea |
AE = adverse event; DB = double-blinded; FSAD = female sexual arousal disorder; FSFI = Female Sexual Function Index; FSFI-d = Female Sexual Function Index desire domain; FSDS-R-13 = Female Sexual Distress Scale–Revised, item 13; FSDS-DAO = Female Sexual Distress Scale–Desire/Arousal/Orgasm; HSDD = hypoactive sexual desire disorder; OL = open-label; PC = placebo-controlled; PK = pharmacokinetic; QS-F = Sexual Quotient Female Version; R = randomized; SSE = satisfying sexual event.
This table includes agents with phase 2 or 3 studies in patients with HSDD.
Evaluated in a single placebo-controlled crossover study; results for each treatment are reported separately.
Bremelanotide (AMAG Pharmaceuticals, Inc, Waltham, MA, USA), a melanocortin receptor agonist formulated as a subcutaneous injection, is currently in late-stage development for the treatment of HSDD (Table 4). In a randomized, placebo-controlled, dose-finding study of women with HSDD and/or female sexual arousal disorder, bremelanotide (1.25 and 1.75 mg) demonstrated significant efficacy vs placebo in measures of sexual desire and arousal and in the number of SSEs.94 The most common adverse events (at doses of 1.25 and 1.75 mg) included nausea (22–24%), flushing (14–17%), and headache (9–14%). In November 2016, it was announced that bremelanotide had met the prespecified co-primary efficacy end points in 2 randomized, placebo-controlled, phase 3 clinical trials of HSDD in premenopausal women.102 In those studies, improvements in FSFI-d and FSDS Desire/Arousal/Orgasm scores were significantly greater with bremelanotide 1.75 mg, administered subcutaneously through an auto-injector before anticipated sexual activity, compared with placebo.95, 96 Coadministration of bremelanotide with alcohol was evaluated in a randomized, placebo-controlled, crossover study of 24 healthy men and women administered intranasal bremelanotide 20 mg with or without ethanol 0.6 g/kg or ethanol alone.97 Overall incidence of adverse events was 75% for bremelanotide coadministered with ethanol, 67% for bremelanotide alone, and 58% for ethanol alone. Compared with bremelanotide alone, small decreases (∼2–6 mm Hg) in mean systolic and diastolic blood pressure were observed for bremelanotide plus ethanol and ethanol alone. An application for FDA approval of bremelanotide for the treatment of HSDD in premenopausal women is expected in early 2018.
Other combination treatments under study include testosterone plus agents that affect neurotransmitters associated with HSDD.99, 100, 103 The combination of sublingual testosterone and sildenafil, a phosphodiesterase type 5 inhibitor (Emotional Brain BV, Almere, The Netherlands), has been evaluated as on-demand treatment in a small, randomized, placebo-controlled study of women with HSDD and low sensitivity to sexual cues.99 Results showed that this combination improved sexual desire and genital arousal. The most common treatment-related adverse events associated with testosterone plus sildenafil were flushing (23.0%) and headache (15.9%). Sublingual testosterone with buspirone (Emotional Brain BV), which is a serotonin 1A receptor agonist with immediate short-term decreases in serotonergic activity, also has been evaluated as on-demand treatment in a small, randomized, placebo-controlled study.100 Results showed that this combination improved sexual functioning in women with HSDD and over-activation of sexual inhibitory mechanisms. Common adverse events included dizziness (11.3%) and lightheadedness (10.3%).
The efficacy of the botanical Tribulus terrestris, which might increase levels of bioavailable testosterone, was investigated in a randomized placebo-controlled study of postmenopausal women with HSDD.101 No significant differences were observed between orally administered T terrestris and placebo on FSFI domain or total scores; however, T terrestris was associated with greater improvement on the non-validated Sexual Quotient Female Version questionnaire, which was developed to assess sexual function in Brazilian women (the study population).
Discussion
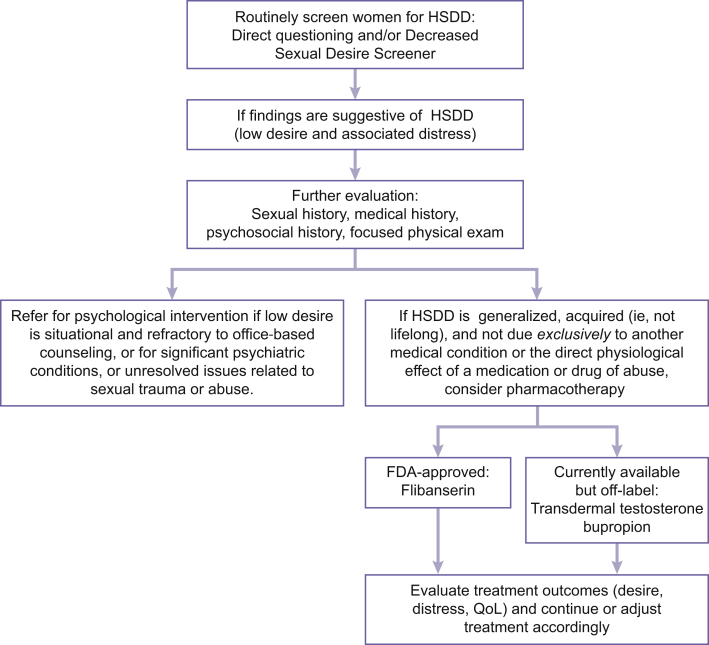
HSDD is a common problem in women, and providers who deliver gynecologic services are uniquely positioned to identify and address this condition. Although each patient requires independent medical judgment, a simple treatment algorithm can assist in the screening, diagnosis, and management of HSDD (Figure 3). Basic screening for HSDD can be accomplished during an office visit—even with time constraints—with more thorough assessment necessary only for women with findings suggestive of HSDD. A patient’s medical history and comorbid conditions (including depression, anxiety, and other conditions that can contribute to low sexual desire) should be reviewed, as should medications, because many, such as selective serotonin reuptake inhibitors and antihypertensives, are associated with low desire. A gynecologic examination could disclose conditions that can negatively affect sexual function (eg, vulvovaginal atrophy resulting in dyspareunia), but only if indicated. Once the suitability of pharmacotherapy is determined, the choice of therapy depends on several factors. Although agents are in development for HSDD, alternative strategies include the use of medications indicated for other conditions (eg, transdermal testosterone, bupropion). Such medications are being used to alleviate the unmet need in postmenopausal women, in addition to availability and cost concerns, because flibanserin is approved by the FDA only for acquired, generalized HSDD in premenopausal women.
Figure 3.
Evaluation and treatment algorithm for HSDD. FDA = US Food and Drug Administration; HSDD = hypoactive sexual desire disorder; QoL = quality of life.
Conclusions
HSDD is a common condition that often goes undiagnosed because of reluctance by patients and health care providers to initiate a discussion of sexual issues. Providers of gynecologic services have the opportunity to screen and counsel patients with HSDD and can work with therapists to develop psychotherapy treatment plans for these women. Pharmacotherapy for HSDD is currently limited to the FDA-approved flibanserin and off-label use of transdermal testosterone and bupropion, but other agents are in development.
Statement of authorship
Category 1
-
(a)Conception and Design
- Anita H. Clayton; Sheryl A. Kingsberg; Irwin Goldstein
-
(b)Acquisition of Data
- Anita H. Clayton; Sheryl A. Kingsberg; Irwin Goldstein
-
(c)Analysis and Interpretation of Data
- Anita H. Clayton; Sheryl A. Kingsberg; Irwin Goldstein
Category 2
-
(a)Drafting the Article
- Anita H. Clayton; Sheryl A. Kingsberg; Irwin Goldstein
-
(b)Revising It for Intellectual Content
- Anita H. Clayton; Sheryl A. Kingsberg; Irwin Goldstein
Category 3
-
(a)Final Approval of the Completed Article
- Anita H. Clayton; Sheryl A. Kingsberg; Irwin Goldstein
Acknowledgments
Technical editorial and medical writing assistance was provided by Nancy Holland, PhD, and Beth Kamp, PharmD, for Synchrony Medical Communications, LLC (West Chester, PA, USA) under the direction of the authors. Funding for this support was provided by Valeant Pharmaceuticals North America LLC.
Footnotes
Conflicts of Interest: Dr Clayton reports receiving research and grant support from Auspex Pharmaceuticals, Axsome Therapeutics, Inc, Forest Research Institute, Inc, Genomind, Janssen Pharmaceuticals, Inc, Palatin Technologies, Inc, SAGE Therapeutics, and Takeda Pharmaceuticals USA, Inc; serving as a consultant for and receiving honoraria from Fabre-Kramer Pharmaceuticals, Inc, Forest Laboratories, Lundbeck, Naurex Inc, Otsuka, Palatin Technologies, Inc, Roche, S1 Biopharma, Inc, Sprout, a division of Valeant Pharmaceuticals North America LLC, and Takeda Global Research and Development; being a stock shareholder of Euthymics Bioscience and S1 Biopharma, Inc; and having royalties and copyrights at Ballantine Books/Random House, the Changes in Sexual Functioning Questionnaire, and Guilford Publications. Dr Kingsberg reports receiving research and grant support from Acerus Pharmaceuticals Corporation, Palatin Technologies, Inc, TherapeuticsMD, Inc, and Valeant Pharmaceuticals North America LLC; receiving consulting fees from Acerus Pharmaceuticals Corporation, Emotional Brain, Endoceutics, Materna Medical Inc, NovoNordisk, Nuelle, Inc, Palatin Technologies, Inc, Pfizer Inc, Sermonix Pharmaceuticals, Shionogi Inc, SST Corporation, Teva Pharmaceutical Industries Ltd, TherapeuticsMD, Inc, and Valeant Pharmaceuticals North America LLC; being a stock shareholder of Viveve; and testifying at the 2015 Food and Drug Administration Advisory Committee meeting regarding flibanserin. Dr Goldstein reports serving on the advisory boards of Nuelle, Inc, The Female Health Company, and Valeant Pharmaceuticals North America LLC; conducting research for Palatin Technologies, Inc, Shionogi, Inc, and Valeant Pharmaceuticals North America LLC; serving on the speakers’ bureau for AMAG Pharmaceuticals, ASCEND Therapeutics US, LLC, and Valeant Pharmaceuticals North America LLC; and acting as a consultant for S1 Biopharma, Inc.
Funding: Valeant Pharmaceuticals North America LLC.
References
- 1.American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. [Google Scholar]
- 2.2017 ICD-10-CM diagnosis code F52.0: hypoactive sexual desire disorder. Available at: http://www.icd10data.com/ICD10CM/codes/F01-F99/F50-F59/F52-/F52.0. Published 2017. Accessed December 7, 2017.
- 3.Goldstein I., Kim N.N., Clayton A.H. Hypoactive sexual desire disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114–128. doi: 10.1016/j.mayocp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Parish S., Hahn S.R. Hypoactive sexual desire disorder: a review of epidemiology, biopsychology, diagnosis, and treatment. Sex Med Rev. 2016;4:103–120. doi: 10.1016/j.sxmr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Shifren J.L., Monz B.U., Russo P.A. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970–978. doi: 10.1097/AOG.0b013e3181898cdb. [DOI] [PubMed] [Google Scholar]
- 6.West S.L., D’Aloisio A.A., Agans R.P. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441–1449. doi: 10.1001/archinte.168.13.1441. [DOI] [PubMed] [Google Scholar]
- 7.Rosen R.C., Connor M.K., Miyasato G. Sexual desire problems in women seeking healthcare: a novel study design for ascertaining prevalence of hypoactive sexual desire disorder in clinic-based samples of U.S. women. J Womens Health (Larchmt) 2012;21:505–515. doi: 10.1089/jwh.2011.3002. [DOI] [PubMed] [Google Scholar]
- 8.Hayes R.D., Dennerstein L., Bennett C.M. Relationship between hypoactive sexual desire disorder and aging. Fertil Steril. 2007;87:107–112. doi: 10.1016/j.fertnstert.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 9.Zeleke B.M., Bell R.J., Billah B. Hypoactive sexual desire dysfunction in community-dwelling older women. Menopause. 2016;24:391–399. doi: 10.1097/GME.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 10.Leiblum S.R., Koochaki P.E., Rodenberg C.A. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS) Menopause. 2006;13:46–56. doi: 10.1097/01.gme.0000172596.76272.06. [DOI] [PubMed] [Google Scholar]
- 11.Biddle A.K., West S.L., D’Aloisio A.A. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12:763–772. doi: 10.1111/j.1524-4733.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- 12.Fisher W., Eardley I., Fuchs M. The desire (desire and its effects on female sexuality including relationships) study: emotional impact of low sexual desire and associated distress in a sample of 5,089 European women. J Sex Med. 2010;7:118–148. [Google Scholar]
- 13.Kingsberg S.A. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt) 2014;23:817–823. doi: 10.1089/jwh.2014.4743. [DOI] [PubMed] [Google Scholar]
- 14.Shifren J.L., Johannes C.B., Monz B.U. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health (Larchmt) 2009;18:461–468. doi: 10.1089/jwh.2008.1133. [DOI] [PubMed] [Google Scholar]
- 15.Bitzer J., Giraldi A., Pfaus J. Sexual desire and hypoactive sexual desire disorder in women. Introduction and overview. Standard operating procedure (SOP Part 1) J Sex Med. 2013;10:36–49. doi: 10.1111/j.1743-6109.2012.02818.x. [DOI] [PubMed] [Google Scholar]
- 16.Kingsberg S.A., Rezaee R.L. Hypoactive sexual desire in women. Menopause. 2013;20:1284–1300. doi: 10.1097/GME.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 17.Clayton A.H. The pathophysiology of hypoactive sexual desire disorder in women. Int J Gynaecol Obstet. 2010;110:7–11. doi: 10.1016/j.ijgo.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Pfaus J.G. Pathways of sexual desire. J Sex Med. 2009;6:1506–1533. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- 19.Stahl S.M. Circuits of sexual desire in hypoactive sexual desire disorder. J Clin Psychiatry. 2010;71:518–519. doi: 10.4088/JCP.10bs06115whi. [DOI] [PubMed] [Google Scholar]
- 20.Holstege G. How the emotional motor system controls the pelvic organs. Sex Med Rev. 2016;4:303–328. doi: 10.1016/j.sxmr.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 11th revision of the International Classification of Diseases, beta draft. Avaialble at: https://icd.who.int/dev11/l-m/en. Published 2016. Accessed October 10, 2017.
- 22.Parish S.J., Goldstein A.T., Goldstein S.W. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions—part II. J Sex Med. 2016;13:1888–1906. doi: 10.1016/j.jsxm.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 24.Balon R., Clayton A.H. Female sexual interest/arousal disorder: a diagnosis out of thin air. Arch Sex Behav. 2014;43:1227–1229. doi: 10.1007/s10508-013-0247-1. [DOI] [PubMed] [Google Scholar]
- 25.Clayton A.H., Derogatis L.R., Rosen R.C. Intended or unintended consequences? The likely implications of raising the bar for sexual dysfunction diagnosis in the proposed DSM-V revisions: 1. For women with incomplete loss of desire or sexual receptivity. J Sex Med. 2012;9:2027–2039. doi: 10.1111/j.1743-6109.2012.02850.x. [DOI] [PubMed] [Google Scholar]
- 26.Nusbaum M.R., Helton M.R., Ray N. The changing nature of women’s sexual health concerns through the midlife years. Maturitas. 2004;49:283–291. doi: 10.1016/j.maturitas.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Abdolrasulnia M., Shewchuk R.M., Roepke N. Management of female sexual problems: perceived barriers, practice patterns, and confidence among primary care physicians and gynecologists. J Sex Med. 2010;7:2499–2508. doi: 10.1111/j.1743-6109.2010.01857.x. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann G. Female sexuality and sexual dysfunction: are we stuck on the learning curve? J Sex Med. 2006;3:639–645. doi: 10.1111/j.1743-6109.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 29.Kingsberg S.A., Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477–486. doi: 10.1097/AOG.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 30.Clayton A.H., Goldfischer E.R., Goldstein I. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD) J Sex Med. 2009;6:730–738. doi: 10.1111/j.1743-6109.2008.01153.x. [DOI] [PubMed] [Google Scholar]
- 31.SMSNA executive office. Decreased Sexual Desire Screener. Available at: http://www.sexhealthmatters.org/resources/decreased-sexual-desire-screener. Published 2016. Accessed December 7, 2017.
- 32.Buster J.E. Managing female sexual dysfunction. Fertil Steril. 2013;100:905–915. doi: 10.1016/j.fertnstert.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Annon J.S. Harper & Row; Hagerstown, MD: 1976. Behavioral treatment of sexual problems: brief therapy. [Google Scholar]
- 34.ter Kuile M.M., Both S., van Lankveld J.J. Cognitive behavioral therapy for sexual dysfunctions in women. Psychiatr Clin North Am. 2010;33:595–610. doi: 10.1016/j.psc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Trudel G., Marchand A., Ravart M. The effect of cognitive behavioral group treatment on hypoactive sexual desire in women. Sex Rel Ther. 2001;16:145–164. [Google Scholar]
- 36.McCabe M.P. Evaluation of a cognitive behavior therapy program for people with sexual dysfunction. J Sex Marital Ther. 2001;27:259–271. doi: 10.1080/009262301750257119. [DOI] [PubMed] [Google Scholar]
- 37.Brotto L.A., Erskine Y., Carey M. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320–325. doi: 10.1016/j.ygyno.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brotto L.A., Basson R. Group mindfulness-based therapy significantly improves sexual desire in women. Behav Res Ther. 2014;57:43–54. doi: 10.1016/j.brat.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Linschoten M., Weiner L., Avery-Clark C. Sensate focus: a critical literature review. J Sex Relatsh Ther. 2016;31:230–247. [Google Scholar]
- 40.van Lankveld J.J. Bibliotherapy in the treatment of sexual dysfunctions: a meta-analysis. J Consult Clin Psychol. 1998;66:702–708. doi: 10.1037//0022-006x.66.4.702. [DOI] [PubMed] [Google Scholar]
- 41.van Lankveld J., Everaerd W., Grotjohann Y. Cognitive-behavioral bibliotherapy for sexual dysfunctions in heterosexual couples: a randomized waiting-list controlled clinical trial in The Netherlands. J Sex Res. 2001;38:51–67. [Google Scholar]
- 42.Mintz L.B., Balzer A.M., Zhao X. Bibliotherapy for low sexual desire: evidence for effectiveness. J Couns Psychol. 2012;59:471–478. doi: 10.1037/a0028946. [DOI] [PubMed] [Google Scholar]
- 43.Wincze J.P., Wesiberg R.B. Guilford Press; New York: 2015. Sexual dysfunction, third edition: a guide for assessment and treatment. [Google Scholar]
- 44.Pyke R.E., Clayton A.H. Psychological treatment trials for hypoactive sexual desire disorder: a sexual medicine critique and perspective. J Sex Med. 2015;12:2451–2458. doi: 10.1111/jsm.13056. [DOI] [PubMed] [Google Scholar]
- 45.Martinho Pereira V., Arias-Carrion O., Machado S. Sex therapy for female sexual dysfunction. Int Arch Med. 2013;6:37. doi: 10.1186/1755-7682-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen R., Brown C., Heiman J. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 47.DeRogatis L., Clayton A., Lewis-D’Agostino D. Validation of the Female Sexual Distress Scale–Revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med. 2008;5:357–364. doi: 10.1111/j.1743-6109.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 48.Joffe H.V., Chang C., Sewell C. FDA approval of flibanserin—treating hypoactive sexual desire disorder. N Engl J Med. 2016;374:101–104. doi: 10.1056/NEJMp1513686. [DOI] [PubMed] [Google Scholar]
- 49.Addyi® (flibanserin) tablets, for oral use [package insert] Sprout Pharmaceuticals, a division of Valeant Pharmaceuticals North America LLC; Bridgewater, NJ: 2016. [Google Scholar]
- 50.Dooley E.M., Miller M.K., Clayton A.H. Flibanserin: from bench to bedside. Sex Med Rev. 2017;5:461–469. doi: 10.1016/j.sxmr.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Stahl S.M., Sommer B., Allers K.A. Multifunctional pharmacology of flibanserin: possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2011;8:15–27. doi: 10.1111/j.1743-6109.2010.02032.x. [DOI] [PubMed] [Google Scholar]
- 52.Kingsberg S.A., Clayton A.H., Pfaus J.G. The female sexual response: current models, neurobiological underpinnings and agents currently approved or under investigation for the treatment of hypoactive sexual desire disorder. CNS Drugs. 2015;29:915–933. doi: 10.1007/s40263-015-0288-1. [DOI] [PubMed] [Google Scholar]
- 53.DeRogatis L.R., Komer L., Katz M. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET Study. J Sex Med. 2012;9:1074–1085. doi: 10.1111/j.1743-6109.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- 54.Thorp J., Simon J., Dattani D. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9:793–804. doi: 10.1111/j.1743-6109.2011.02595.x. [DOI] [PubMed] [Google Scholar]
- 55.Katz M., DeRogatis L.R., Ackerman R. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10:1807–1815. doi: 10.1111/jsm.12189. [DOI] [PubMed] [Google Scholar]
- 56.Simon J.A., Kingsberg S.A., Shumel B. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21:633–640. doi: 10.1097/GME.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 57.Portman D.J., Brown L., Yuan J., Kissling R., Kingsberg S.A. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834–842. doi: 10.1016/j.jsxm.2017.03.258. [DOI] [PubMed] [Google Scholar]
- 58.Goldfischer E.R., Breaux J., Katz M. Continued efficacy and safety of flibanserin in premenopausal women with hypoactive sexual desire disorder (HSDD): results from a randomized withdrawal trial. J Sex Med. 2011;8:3160–3172. doi: 10.1111/j.1743-6109.2011.02458.x. [DOI] [PubMed] [Google Scholar]
- 59.Jayne C., Simon J.A., Taylor L.V. Open-label extension study of flibanserin in women with hypoactive sexual desire disorder. J Sex Med. 2012;9:3180–3188. doi: 10.1111/j.1743-6109.2012.02942.x. [DOI] [PubMed] [Google Scholar]
- 60.Clayton AH, Croft HA, Yuan J, et al. Safety of flibanserin in women treated for depression with an SSRI or SNRI: a 12-week, randomized, placebo-controlled study. Presented at: 24th Annual Congress of Women’s Health; April 14–17, 2016; Washington, DC, USA.
- 61.Stevens D.M., Weems J.M., Brown L. The pharmacodynamic effects of combined administration of flibanserin and alcohol. J Clin Pharm Ther. 2017;42:598–606. doi: 10.1111/jcpt.12563. [DOI] [PubMed] [Google Scholar]
- 62.Kay G.G., Hochadel T., Sicard E. Next-day residual effects of flibanserin on simulated driving performance in premenopausal women. Hum Psychopharmacol. 2017;32:e2603. doi: 10.1002/hup.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kupferer E, Kagan R, Brown L, et al. Use of flibanserin with hormonal contraceptives: analysis of phase 1 pharmacokinetic and phase 3 clinical studies. Presented at: 19th Annual Conference of Nurse Practitioners in Women’s Health (NPWH); September 28–October 1, 2016; New Orleans, LA, USA.
- 64.Noll J, Brown L, Yuan J, Kissling R. Effect of flibanserin on the pharmacokinetics of a combined ethinylestradiol/levonorgestrel oral contraceptive in healthy premenopausal women. Presented at: 24th Annual Congress of Women’s Health; April 14–17, 2016; Washington, DC, USA.
- 65.Troconiz I.F., Boland K., Staab A. Population pharmacokinetic/pharmacodynamic model for the sedative effects of flibanserin in healthy volunteers. Pharm Res. 2012;29:1518–1529. doi: 10.1007/s11095-011-0648-6. [DOI] [PubMed] [Google Scholar]
- 66.Sprout Pharmaceuticals, a division of Valeant Pharmaceuticals North America LLC Addyi risk evaluation and mitigation strategy (REMS) https://addyirems.com/AddyiUI/rems/home.action Available at:
- 67.Achilli C., Pundir J., Ramanathan P. Efficacy and safety of transdermal testosterone in postmenopausal women with hypoactive sexual desire disorder: a systematic review and meta-analysis. Fertil Steril. 2017;107:475–482.e415. doi: 10.1016/j.fertnstert.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 68.Braunstein G.D., Sundwall D.A., Katz M. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:1582–1589. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- 69.Buster J.E., Kingsberg S.A., Aguirre O. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005;105:944–952. doi: 10.1097/01.AOG.0000158103.27672.0d. [DOI] [PubMed] [Google Scholar]
- 70.Simon J., Braunstein G., Nachtigall L. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226–5233. doi: 10.1210/jc.2004-1747. [DOI] [PubMed] [Google Scholar]
- 71.Davis S.R., van der Mooren M.J., van Lunsen R.H. Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Menopause. 2006;13:387–396. doi: 10.1097/01.gme.0000179049.08371.c7. [DOI] [PubMed] [Google Scholar]
- 72.Shifren J.L., Davis S.R., Moreau M. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770–779. doi: 10.1097/01.gme.0000243567.32828.99. [DOI] [PubMed] [Google Scholar]
- 73.Davis S.R., Moreau M., Kroll R. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005–2017. doi: 10.1056/NEJMoa0707302. [DOI] [PubMed] [Google Scholar]
- 74.Panay N., Al-Azzawi F., Bouchard C. Testosterone treatment of HSDD in naturally menopausal women: the ADORE study. Climacteric. 2010;13:121–131. doi: 10.3109/13697131003675922. [DOI] [PubMed] [Google Scholar]
- 75.Waldman T., Shufelt C.L., Braunstein G.D. Safety and efficacy of transdermal testosterone for treatment of hypoactive sexual desire disorder. Clin Invest. 2012;2:423–432. [Google Scholar]
- 76.Segraves R.T., Clayton A., Croft H. Bupropion sustained release for the treatment of hypoactive sexual desire disorder in premenopausal women. J Clin Psychopharmacol. 2004;24:339–342. doi: 10.1097/01.jcp.0000125686.20338.c1. [DOI] [PubMed] [Google Scholar]
- 77.Segraves R.T., Croft H., Kavoussi R. Bupropion sustained release (SR) for the treatment of hypoactive sexual desire disorder (HSDD) in nondepressed women. J Sex Marital Ther. 2001;27:303–316. doi: 10.1080/009262301750257155. [DOI] [PubMed] [Google Scholar]
- 78.Reed B.G., Bou Nemer L., Carr B.R. Has testosterone passed the test in premenopausal women with low libido? A systematic review. Int J Womens Health. 2016;8:599–607. doi: 10.2147/IJWH.S116212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shifren J.L., Braunstein G.D., Simon J.A. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 80.Kingsberg S.A., Simon J.A., Goldstein I. The current outlook for testosterone in the management of hypoactive sexual desire disorder in postmenopausal women. J Sex Med. 2008;5:182–193. doi: 10.1111/j.1743-6109.2008.00961.x. quiz 193. [DOI] [PubMed] [Google Scholar]
- 81.Reis S.L., Abdo C.H. Benefits and risks of testosterone treatment for hypoactive sexual desire disorder in women: a critical review of studies published in the decades preceding and succeeding the advent of phosphodiesterase type 5 inhibitors. Clinics (Sao Paulo) 2014;69:294–303. doi: 10.6061/clinics/2014(04)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shifren J.L. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147–1149. doi: 10.1097/GME.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 83.Davis S.R., Worsley R., Miller K.K. Androgens and female sexual function and dysfunction—findings from the Fourth International Consultation of Sexual Medicine. J Sex Med. 2016;13:168–178. doi: 10.1016/j.jsxm.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 84.Spark R.F. Intrinsa fails to impress FDA advisory panel. Int J Impot Res. 2005;17:283–284. doi: 10.1038/sj.ijir.3901308. [DOI] [PubMed] [Google Scholar]
- 85.Kingsberg S.A. The testosterone patch for women. Int J Impot Res. 2005;17:465–466. doi: 10.1038/sj.ijir.3901375. [DOI] [PubMed] [Google Scholar]
- 86.Clayton A.H., Hamilton D.V. Female sexual dysfunction. Psychiatr Clin North Am. 2010;33:323–338. doi: 10.1016/j.psc.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 87.Nastri C.O., Lara L.A., Ferriani R.A. Hormone therapy for sexual function in perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2013;6:CD009672. doi: 10.1002/14651858.CD009672.pub2. [DOI] [PubMed] [Google Scholar]
- 88.Santoro N., Worsley R., Miller K.K. Role of estrogens and estrogen-like compounds in female sexual function and dysfunction. J Sex Med. 2016;13:305–316. doi: 10.1016/j.jsxm.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 89.Segraves R.T., Kavoussi R., Hughes A.R. Evaluation of sexual functioning in depressed outpatients: a double-blind comparison of sustained-release bupropion and sertraline treatment. J Clin Psychopharmacol. 2000;20:122–128. doi: 10.1097/00004714-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Dobkin R.D., Menza M., Marin H. Bupropion improves sexual functioning in depressed minority women: an open-label switch study. J Clin Psychopharmacol. 2006;26:21–26. doi: 10.1097/01.jcp.0000194623.07611.90. [DOI] [PubMed] [Google Scholar]
- 91.Clayton A.H., Croft H.A., Horrigan J.P. Bupropion extended release compared with escitalopram: effects on sexual functioning and antidepressant efficacy in 2 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry. 2006;67:736–746. doi: 10.4088/jcp.v67n0507. [DOI] [PubMed] [Google Scholar]
- 92.Clayton A.H., Pradko J.F., Croft H.A. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63:357–366. doi: 10.4088/jcp.v63n0414. [DOI] [PubMed] [Google Scholar]
- 93.Thase M.E., Clayton A.H., Haight B.R. A double-blind comparison between bupropion XL and venlafaxine XR: sexual functioning, antidepressant efficacy, and tolerability. J Clin Psychopharmacol. 2006;26:482–488. doi: 10.1097/01.jcp.0000239790.83707.ab. [DOI] [PubMed] [Google Scholar]
- 94.Clayton A.H., Althof S.E., Kingsberg S. Bremelanotide for female sexual dysfunctions in premenopausal women: a randomized, placebo-controlled dose-finding trial. Womens Health (Lond) 2016;12:325–337. doi: 10.2217/whe-2016-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clayton A.H., Kingsberg S.A., Jordan E. 2017. Efficacy of the investigational drug bremelanotide for hypoactive sexual desire disorder (HSDD): results from the RECONNECT study. Presented at: Annual Meeting of the American Society of Clinical Psychopharmacology; May 30, 2017. Miami, FL, USA. [Google Scholar]
- 96.DeRogatis L, Althof S, Clayton A, et al. Changes in arousal and desire in bremelanotide RECONNECT study. Presented at: Annual Meeting of the International Society for the Study of Women’s Sexual Health (ISSWSH); February 23–26, 2017; Atlanta, GA, USA.
- 97.Clayton A.H., Lucas J., DeRogatis L.R. Phase I randomized placebo-controlled, double-blind study of the safety and tolerability of bremelanotide coadministered with ethanol in healthy male and female participants. Clin Ther. 2017;39:514–526.e514. doi: 10.1016/j.clinthera.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 98.Pyke RE, Katz M, Segraves RT, et al. Phase IIa study of a proprietary combination of bupropion and trazodone for hypoactive sexual desire disorder (HSDD) in premenopausal women: novel responder and remitter results. Presented at: Annual Meeting of the American Society of Clinical Psychopharmacology; June 22–25, 2015; Miami, FL, USA.
- 99.Poels S., Bloemers J., van Rooij K. Toward personalized sexual medicine (part 2): testosterone combined with a PDE5 inhibitor increases sexual satisfaction in women with HSDD and FSAD, and a low sensitive system for sexual cues. J Sex Med. 2013;10:810–823. doi: 10.1111/j.1743-6109.2012.02983.x. [DOI] [PubMed] [Google Scholar]
- 100.van Rooij K., Poels S., Bloemers J. Toward personalized sexual medicine (part 3): testosterone combined with a serotonin1A receptor agonist increases sexual satisfaction in women with HSDD and FSAD, and dysfunctional activation of sexual inhibitory mechanisms. J Sex Med. 2013;10:824–837. doi: 10.1111/j.1743-6109.2012.02982.x. [DOI] [PubMed] [Google Scholar]
- 101.de Souza K.Z., Vale F.B., Geber S. Efficacy of Tribulus terrestris for the treatment of hypoactive sexual desire disorder in postmenopausal women: a randomized, double-blinded, placebo-controlled trial. Menopause. 2016;23:1252–1256. doi: 10.1097/GME.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 102.Bremelanotide meets co-primary endpoints in Palatin’s phase 3 trials for hypoactive sexual desire disorder [press release] Palatin Technologies; Cranbury, NJ: November 1, 2016. [Google Scholar]
- 103.Poels S., Bloemers J., van R.K. Two novel combined drug treatments for women with hypoactive sexual desire disorder. Pharmacol Biochem Behav. 2014;121:71–79. doi: 10.1016/j.pbb.2014.02.002. [DOI] [PubMed] [Google Scholar]