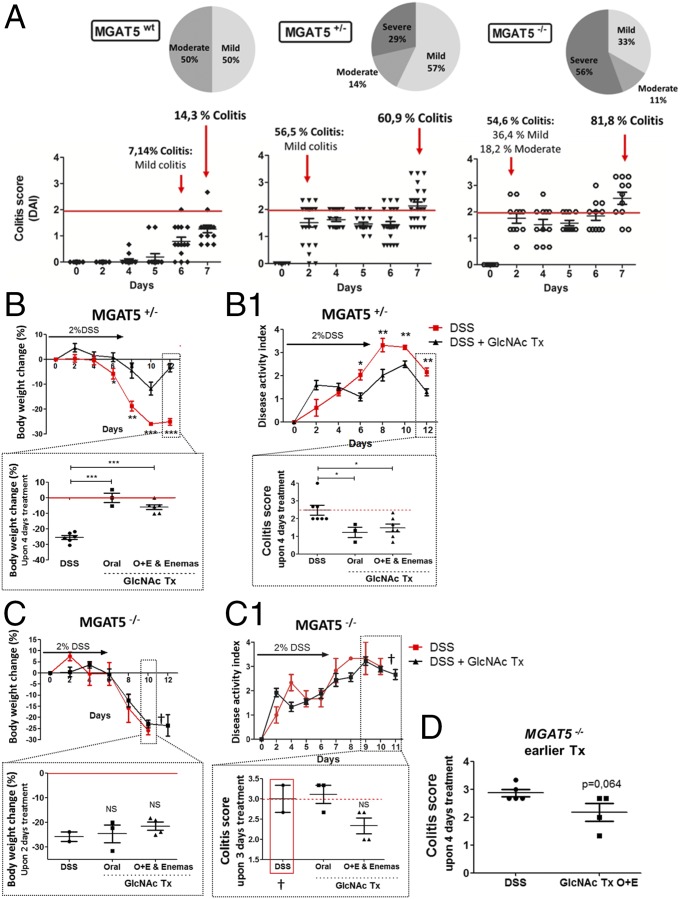
Fig. 6.
MGAT5 null or heterozygous mice develop early-onset colitis and an increase in disease severity that is suppressed by GlcNAc treatment. (A) Evaluation of colitis onset and disease severity in MGAT5 null or heterozygous mice: C57BL/6 WT (n = 14), MGAT5+/− (n = 23), and MGAT5−/− (n = 11) mice. Active disease was defined when animals showed a DAI of ≥2, and three stages of severity were defined: mild (≥2 and <2.5), moderate (≥2.5 and <3), and severe (≥3). Average results of body weight change (B and C) and DAI (B1 and C1) of MGAT5+/− (n = 23) and MGAT5−/− (n = 9) mice, respectively, randomly distributed in controls and GlcNAc treatment groups are shown. DSS-induced colitis (DSS) vs. DSS treated with GlcNAc treatment (DSS + GlcNAc Tx). Animals showing severe signs of disease were euthanized (†). (B and B1, Insets) Discrimination of the efficiency of GlcNAc treatment (colitis scores) with different routes of administration upon 4 d of treatment. Graphs correspond to the mean ± SEM of 17 animals (three to seven animals per route of administration). Student’s t test (B and B1) and one-way ANOVA with Bonferroni postcorrection (B and B1, Insets): *P ≤ 0.05*; **P ≤ 0.01; ***P ≤ 0.001. Body weight changes of MGAT5−/− mice treated through different routes vs. nontreated upon 2 d of treatment (C) and DAI scores of MGAT5−/− mice treated (n = 7) vs. nontreated (n = 2) (C1) are shown. (C and C1, Insets) Discrimination of the efficiency of GlcNAc treatment (colitis scores) with different routes of administration upon 3 d of treatment. (D) Evaluation of the impact of early oral route (O) + enema route (E) GlcNAc treatment (starting on the second day of disease onset: 5–6 d after DSS induction) on the colitis scores (DAI of animals per group) of MGAT5−/− mice, comparing DSS (n = 5) with GlcNAc treated mice (n = 4).