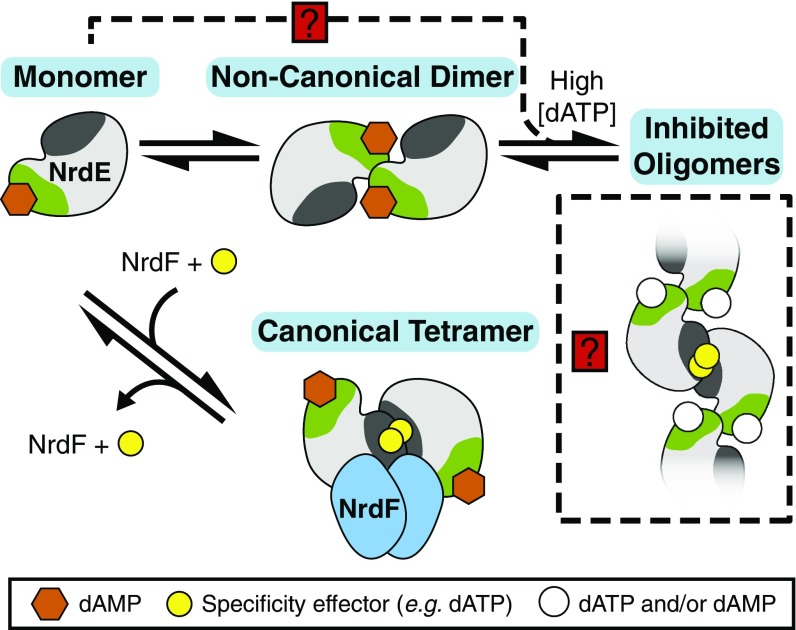
Fig. 8.
A model describing the overall activity allosteric regulation of the B. subtilis class Ib RNR. NrdE, when loaded with dAMP (orange hexagons), exists in an equilibrium between monomer and the noncanonical dimer. The monomer pool is capable of forming a canonical dimer and becoming active for NDP reduction when it binds to Mn(III)2–Y• NrdF with a second monomer, and in the presence of the specificity effector (yellow circles) it potentiates α2β2 complex formation. At high dATP concentrations, noncanonical dimers and possibly monomers (shown as a dashed line with a red box and a question mark) can associate into inhibited, extended oligomeric structures involving the canonical dimer interface, shown in dark gray. The N-terminal dATP-binding site of the oligomer is represented by an open circle suggesting two possible cases: in one, dATP displaces dAMP, and in a second, both dAMP and dATP bind.