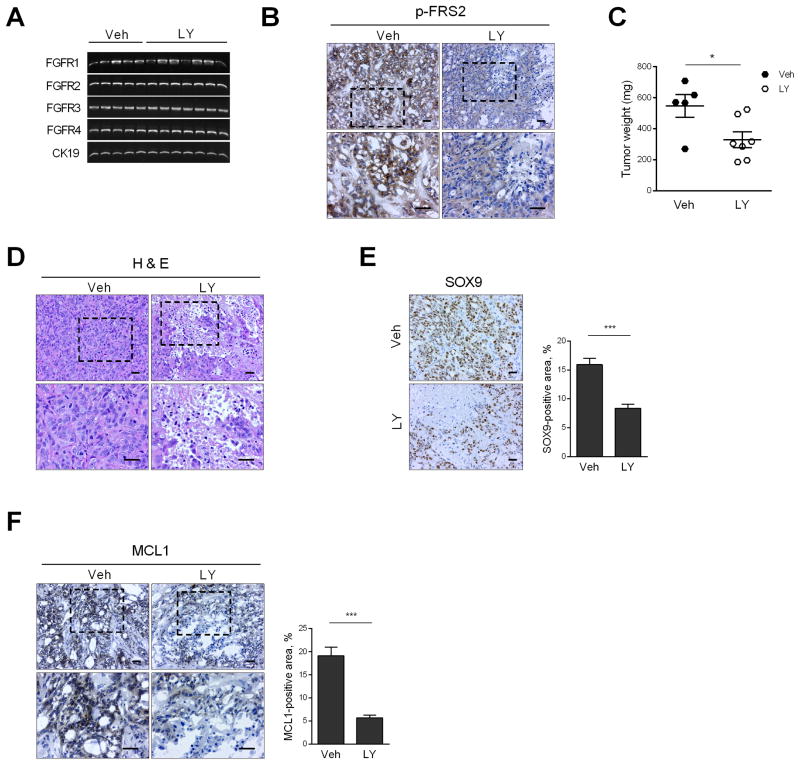
Fig. 6. FGFR inhibition reduces patient-derived xenograft (PDX) tumor weight by inducing necrosis.
CCA PDX was implanted heterotopically in NOD/SCID mice. When the tumor diameter reached 1 cm, mice were randomized to receive either vehicle (Veh, n=5) or LY2874455 (LY, 3 mg/kg body weight, n=7) via oral gavage for 1 week. (A) At the end of the study, tumor RNA was extracted and RT-PCR was performed for FGFR1, 2, 3, 4 and CK19 (loading control). (B) Immunohistochemistry for p-FRS2 in tumors of vehicle and LY-treated mice. Top panel: 20× objective; bottom panel: 40× objective. Scale bar: 20 μm. (C) Tumor weight in vehicle and LY-treated mice. *p < 0.05. (D) Hematoxylin and eosin-stained tumors in vehicle and LY-treated mice. Scale bar: 20 μm. (E) Immunohistochemistry for CCA marker SOX9 in vehicle and LY-treated mice. Scale bar: 20 μm. SOX9-positive area was assessed by morphometry (20× objective). ***p < 0.001. (F) Immunohistochemistry for MCL1 in vehicle and LY-treated mice. Top panel: 20× objective; bottom panel: 40× objective. Scale bar: 20 μm. MCL1-positive area was assessed by morphometry (20× objective). ***p < 0.001. All statistical significance was determined using the two-tailed t test.