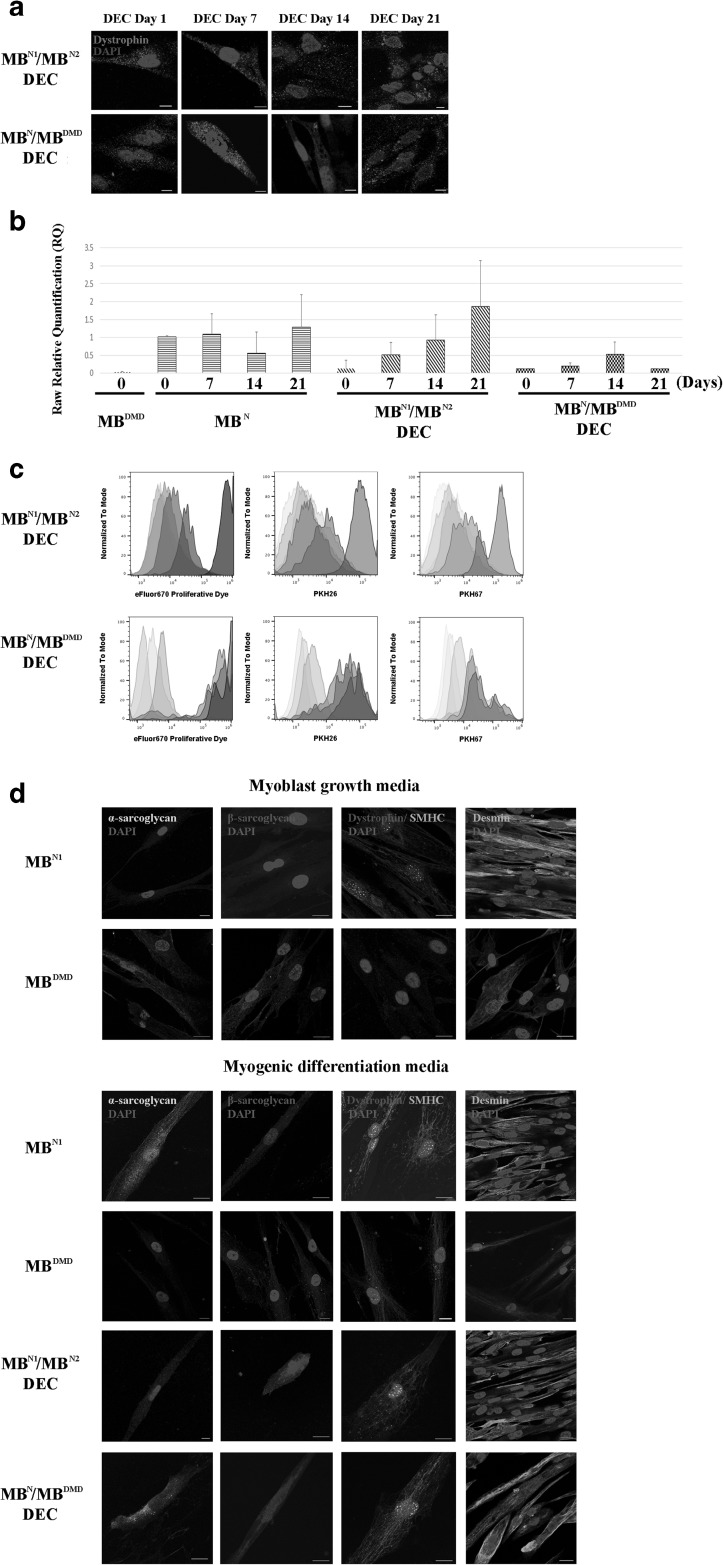
Fig. 3.
Human DEC lines of both, normal (MBN1/MBN2) and normal and DMD donor (MBN/MBDMD) origin, maintain dystrophin expression, proliferation properties and undergo myogenic differentiation in culture. (a) Representative immunofluorescence images of dystrophin expression (magenta) in MBN1/MBN2 and MBN/MBDMD DEC in vitro up to 21 days after fusion (n = 4, magnification 400X, scale bar 10 µm). (b) Dystrophin expression in cultured MBN1/MBN2 and MBN/MBDMD DEC up to 21 days after fusion quantified by Taqman PCR (n = 3, mean ± SD). (c) Flow cytometry analysis confirming proliferation of MBN1/MBN2 (upper row) and MBN/MBDMD (lower row) DEC up to day 21 post-fusion. Decrease in the intensity of eFluor 670 Proliferative Dye correlated with decrease in the intensity of double PKH67/PKH26 staining used to confirm DEC fusion. (d) Immunofluorescence images of MBN1/MBN2 and MBN/MBDMD DEC expressing sarcolemmal glycoproteins, alpha-sarcoglycan (yellow), beta-sarcoglycan (violet) and dystrophin (red) co-expressed with the motor protein skeletal myosin heavy chain (SMHC, green), and fusion protein desmin (green) after 7 days of stimulation in the myogenic differentiation media. Upper panel: Immunofluorescence images of normal (MBN) and DMD-affected (MBDMD) undifferentiated myoblast controls before fusion confirming lack of dystrophin expression in MBDMD and a diffused distribution of SMHC in both MBN and MBDMD. Lower panel: Immunofluorescence images of MBN, MBDMD, MBN1/MBN2 and MBN/MBDMD DEC after 7-day stimulation in the myogenic differentiation medium confirming expression of dystrophin–glycoprotein complex (DGC) in MBN/MBDMD DEC after fusion suggesting restoration of functional DGC. Uniform distribution of alpha-sarcoglycan and beta-sarcoglycan was observed after 7 days of induced differentiation further confirming progression of myogenic maturation. Nuclei were counterstained with DAPI (blue). Scale bar 20 µm