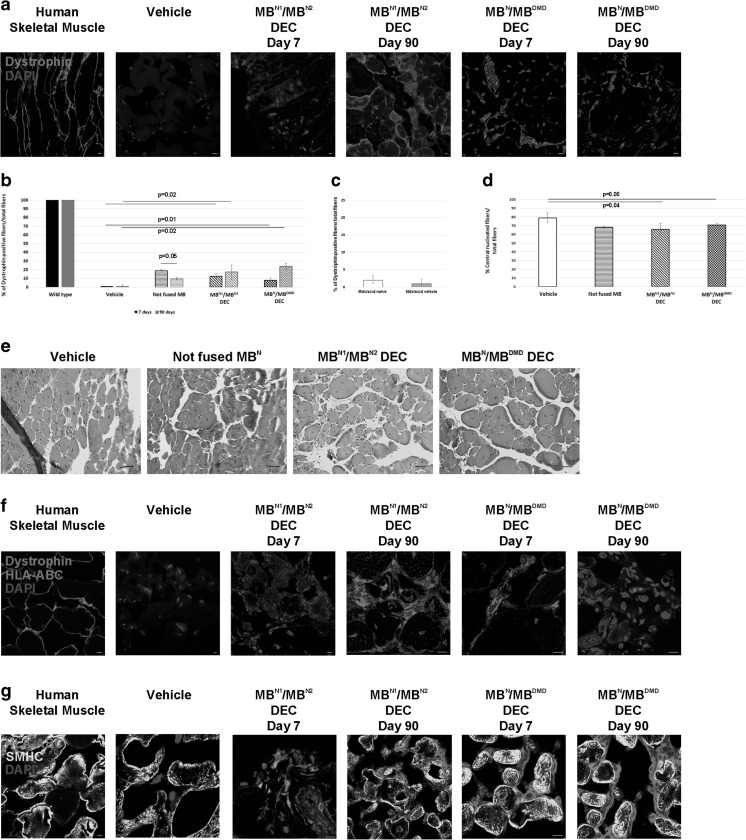
Fig. 4.
Both DEC lines (MBN1/MBN2 and MBN/MBDMD) engraft, differentiate into skeletal muscles and maintain dystrophin expression up to 90 days after intramuscular transplantation to the gastrocnemius muscle (GM) of the mdx/scid mouse. (a) Representative immunofluorescence images presenting restoration of dystrophin expression (magenta) in the GM of mdx/scid mice injected with MBN1/MBN2 and MBN/MBDMD DEC. (b) Quantification of dystrophin expressing muscle fibers for both DEC lines: MBN1/MBN2 (12.3%± 2.5 at day 7 and 17.27%± 8.05 at day 90, n = 6) and MBN/ MBDMD (7.42%± 3.25 at day 7 and 23.79%±3.82 at day 90, n = 6) after DEC transplant to the GM of mdx/scid mice (mean ± SD, p < 0.05 vs. vehicle). Dystrophin positive fiber counts were normalized to the total nuclei count within the region of interest (12ROI/sample). (c) Quantification of the dystrophin positive myocytes in GM of naïve and vehicle injected mdx/scid mice confirming low number (2%) of revertant fibers. Dystrophin expression in GM of age-matched naïve (n = 3) and vehicle-injected (n = 4) mdx/scid mice (mean ± SDM). (d) Quantification of the number of central nucleated muscle fibers in the GM injected with DEC indicates improvement in the dystrophic muscle (mean ± SD, p ≤ 0.05). (e) Representative images of hematoxylin & eosin (H&E) stained GM cross-sections of mdx/scid at 90 days after injection with vehicle, not fused MBN, MBN1/MBN2 and MBN/MBDMD DEC, quantified for number of centrally nucleated muscle fibers. Images of five regions of interest (ROI) were used in three non-serial sections of each treated GM harvested from six animals/group (scale bar 50µ m). GM injected with DEC lines (MBN1/MBN2 DEC, MBN/MBDMD DEC) and not-fused MBN showed reduced number of muscle fibers with centrally located nuclei when compared to vehicle treated animals (controls), indicating restoration towards mature muscle fibers. (n = 6, mean ± SD, p < 0.05, One –way ANOVA). (f) Representative fluorescence images of dystrophin (green) expression co-localized with the expression of HLA-ABC (red) detecting human origin of the dystrophin positive muscle fibers (magnification 400X, scale bar 10µ m). (g) Representative fluorescence images of differentiated myofibers of DEC origin in the GM of mdx/scid mice detected by co-localization of human specific skeletal myosin heavy chain (SMHC, yellow) and HLA-ABC staining (red); (Magnification 400X, scale bar 10µ m)