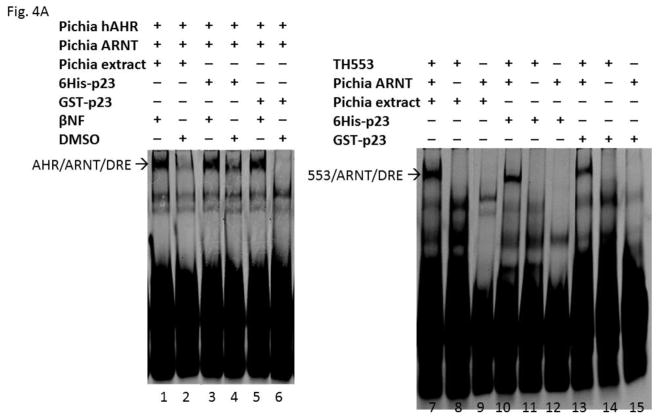
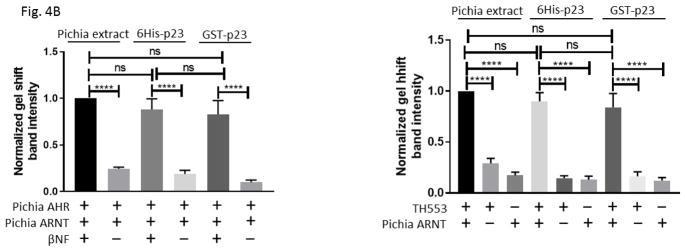
Fig. 4.
GST-p23 promoted the formation of the AHR gel shift complex in vitro. (A) Gel shift assay images showing that GST fusion of p23 (GST-p23) and histidine fusion of p23 (6His-p23) promoted formation of the βNF-dependent AHR/ARNT/DRE complex formation (left panel, lanes 1–6). AHR and ARNT are full length human proteins expressed in Pichia. Both p23 fusions also promoted the formation of the ligand-independent AHR/ARNT/DRE complex with TH553 as the thioredoxin fusion of the human AHR aa1-295, a constitutively active AHR construct (right panel, lanes 7–15). Samples with 6His-p23 and Pichia extract were controls. Arrows indicate the two different AHR gel shift complexes. The experiment was repeated and the gel shift band intensity was quantified using a LI-COR CLx Odyssey imager with either AHR/ARNT/βNF/Pichia extract/DRE or TH553/ARNT/Pichia extract to be arbitrarily set as one. The graph was plotted in B (n = 4, means ± SD).