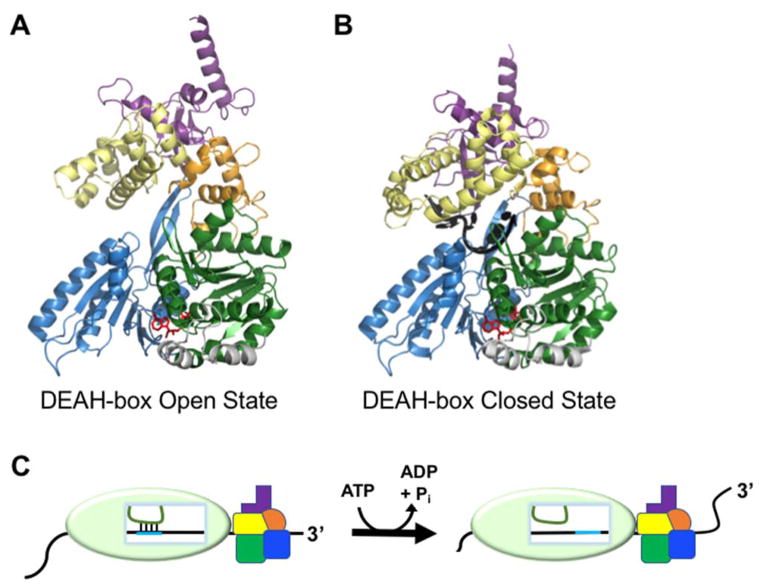
Figure 3.
Remodeling of internal RNA duplexes by DEAH-box proteins. (A) Crystal structure of Prp43 with bound ADP-BeFx showing the open conformation, which facilitates RNA loading (PDB: 5LTK)(57). Domains are colored as in Fig. 1. (B) Crystal structure of the closed conformation of Prp43. The structure includes bound ADP-BeFx and U7 RNA and reflects the conformation that follows RNA loading (PDB: 5LTA)(57). (C) Model for DEAH-box helicase-catalyzed disruption of an RNA duplex within a larger RNP complex by molecular winching (68). Translocation of the protein against the RNP surface results in movement of the RNA (black) relative to the interior of the RNAP. Because its base-pairing partner (green) is held in place relative to the RNP, the movement of the black RNA results in disruption of the base pairs between the two RNAs.