Abstract
We report the first application of pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a rescue therapy before palliative D2 gastrectomy combined with liver metastasectomy performed in a 49-year-old woman with peritoneal carcinomatosis who was primarily diagnosed with and underwent surgery for a Krukenberg tumor. The PIPAC procedure was performed with the use of cisplatin at 7.5 mg/m2 and doxorubicin at 1.5 mg/m2 for 30 min at 37 °C. Eight weeks after the PIPAC procedure, the patient underwent open classic D2 gastrectomy with the creation of a Roux-en-Y anastomosis (RNY) combined with liver metastasectomy. The patient underwent the classic protocol for chemotherapy combined with Xeloda. The patient felt better and returned to her daily activities. Multicenter data should be gathered to confirm the usefulness of PIPAC as a rescue or neoadjuvant supportive therapy in a very select group of patients who have been recently qualified to undergo classic chemotherapy or standard oncologic surgical procedures.
Keywords: Peritoneal carcinomatosis, Pressurized intraperitoneal aerosol chemotherapy, Neoadjuvant therapy, Gastric cancer, Krukenberg tumor
Core tip: The Krukenberg tumor (KT) is very often misdiagnosed as primary ovarian cancer and may be occasionally diagnosed during a clinical work-up. The fast implementation of effective treatment is always necessary. This case might contribute to future confirmation of the usefulness of pressurized intraperitoneal aerosol chemotherapy as a rescue or neoadjuvant, supportive form of therapy in a very select group of patients. This clinical development might be particularly important for patients with a KT presentation of gastric cancer who have been recently qualified to undergo classic chemotherapy or standard oncologic surgical procedures.
INTRODUCTION
Gastric cancer (GC) is one of the leading causes of cancer-related death worldwide, despite the fact that knowledge about its etiology, diagnostics, systemic chemotherapy and surgical techniques have significantly developed and improved during the last three decades[1-4]. The most problematic issue concerning the clinical management of GC is diagnosis at an advanced, and often metastasized, stage[5]. This circumstance is explained by the fact that local symptoms occur only at an advanced stage. Apart from extensive local tumor growth rendering the patient ineligible for curative resection, peritoneal carcinomatosis, which is synchronous in approximately 10% of patients, limits therapeutic options in this subset of patients[6-11].
Another metastatic site, other than the peritoneum, is the ovaries; tumors at these sites are referred to as Krukenberg tumors (KTs). These tumors are very often misdiagnosed as primary ovarian cancer and may be occasionally diagnosed during a clinical work-up for abdominal tenderness in the lower abdomen[12,13]. The KT is described mainly as a rare metastatic tumor of the ovary that originates from the gastrointestinal tract (stomach - 76%; colorectum - 4%, biliary system - 3%; appendix - 3%) but can also originate from breast (4%) and from other miscellaneous sites such as the pancreas, uterus, cervix, or urinary bladder[14,15]. KT is considered a late-stage disease, and despite growing clinical knowledge, there are still many controversies regarding standardized treatment protocols for this subset of patients[16]. To date, there are no universally accepted and recommended prognostic factors for KT treatment that indicate the superiority of one particular surgical algorithm or chemotherapeutic regimen over another[17,18].
We report the first case in the recent and past literature of the application of pressurized intraperitoneal aerosol chemotherapy (PIPAC) as neoadjuvant therapy before palliative D2 gastrectomy combined with liver metastasectomy performed in a patient who was with primarily diagnosed and underwent surgery for a KT.
CASE REPORT
A 49-year-old woman with several co-morbidities (Table 1) was admitted in February 2017 to another hospital with ascites and abdominal masses. Abdominal ultrasound showed bilateral ovarian tumors, ascites and suspicion of peritoneal metastases, suggesting locally advanced ovarian cancer. An exploratory laparotomy was performed by the gynecology team, confirming the presence of two ovarian masses (left: 8 cm × 6 cm, right 12 cm × 8 cm), diffuse peritoneal metastasis (peritoneal carcinomatosis index - PCI = 19) and ascites (volume = 120 mL). Bilateral hysterectomy was performed in combination with bilateral adnexectomy, omentectomy, appendectomy and pelvic lymphadenectomy. The intraoperative situs was evaluated by the gastrointestinal surgeon who confirmed that complete cytoreduction was not possible.
Table 1.
Coexisting diseases - data from the patient’s medical history report
| Diabetes - Type 1 |
| Hypothyroidism |
| Paroxysmal atrial fibrilation |
| Hypertension |
| Rheumatoid arthritis |
Recovery was uneventful. Histology revealed signet ring tumor cells arranged singly, in cords or in nests within cellular ovarian stroma (Figure 1A and B). Additional staining revealed CK7(+), CK20(-), CDX2(+) and CA125(-) status, suggesting a primary tumor originating from the upper gastrointestinal (GI-) tract. Postoperative upper GI endoscopy showed a mucin-positive, poorly differentiated adenocarcinoma located in the antral mucosa (Figure 1C and D). Staging was completed with an abdominal CT scan that showed a superficially located metastasis in liver segment 5 (Figure 2).
Figure 1.
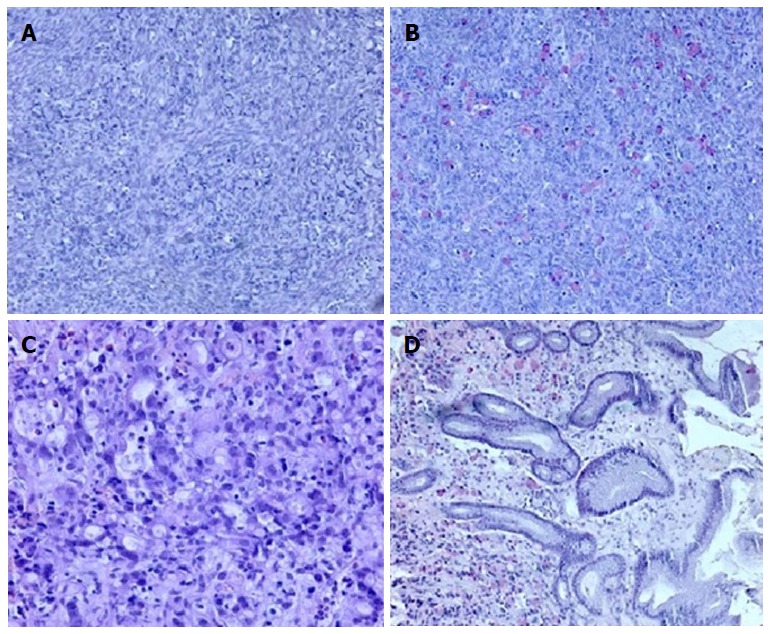
Histological ovary assay. A: Signet ring of tumor cell infiltration within the ovarian stroma (10 ×, HE); B: Mucicarmine staining highlights the presence of mucin in the cytoplasm (10 ×). Histopathologic evaluation of antral mucosa showing (C) poorly differentiated carcinoma infiltration with signet ring cells (20 ×, HE) and (D) mucicarmine staining of mucin-positive cells in the gastric mucosa (10 ×).
Figure 2.
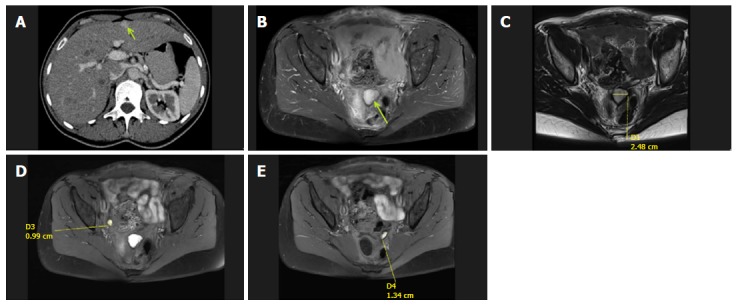
Abdominal computed tomography and abdominopelvic magnetic resonance scans. A: Post-contrast computed tomography image in the portal venous phase showing a hyperintense enhancing lesion in segment V (isodense in the native phase) of the liver, which was diagnosed as a superficially located suspicious metastatic lesion; B-E: Abdominopelvic magnetic resonance scans with evident masses and suspicious nodules.
The patient was referred in April 2017 to our tertiary center for further therapy.
At admission, the patient had reduced general condition (ECOG - 2) and in reduced nutritional status (BMI of 18.37 and weight loss identified in the nutritional anamnesis). Laboratory tests were within normal limits. The patient was presented to the multidisciplinary tumor board, and a systemic combination chemotherapy combined with intraperitoneal chemotherapy with cisplatin and doxorubicin (as a pressurized aerosol, PIPAC C/D) was recommended, with palliative intent. The patient gave voluntary and informed consent to the planned treatment, and the study was performed in accordance with the precepts established by the declaration of Helsinki.
The PIPAC procedure was first performed in May 2017, according to standard protocols described by Hubner et al[19]. Shortly thereafter, after a 12 mmHg capnoperitoneum has been established, two trocars were inserted, and a staging laparoscopy was performed. After confirmation of the tightness of the abdomen, a solution of low-dose cisplatin (7.5 mg/m2 BSA) and doxorubicin (1.5 mg/m2 BSA) diluted in 200 mL of saline solution was aerosolized at a pressure of 12 mmHg and a temperature of 37 °C into the abdomen using a CE-certified nebulizer (Capnopen®, Capnomed, Villingendorf, Germany). After 30 min of application, the toxic aerosol was safely removed via a closed aerosol waste system (CAWS). The diagram displaying the PIPAC procedure is presented in Figure 3. The PIPAC procedure was tolerated very well, and no postoperative complications were noted.
Figure 3.
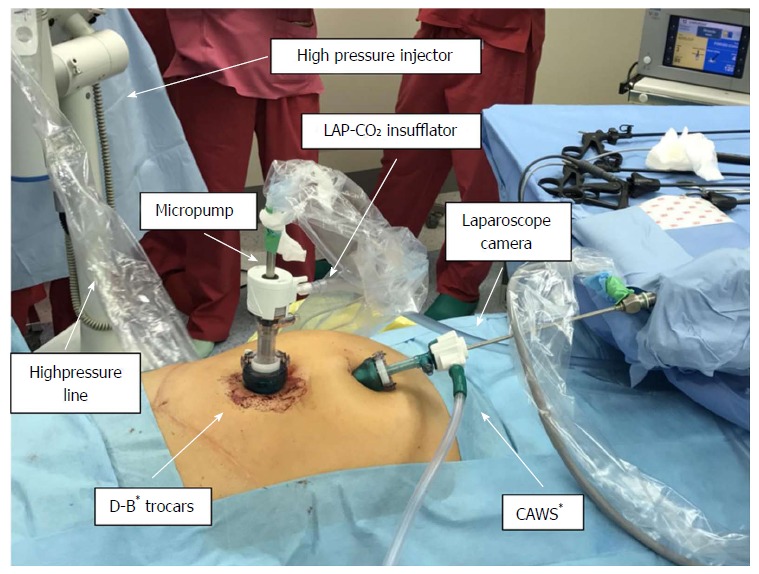
Schematic presentation of the pressurized intraperitoneal aerosol chemotherapy procedure. The procedure is performed under general anesthesia and based on standard diagnostic laparoscopy procedures. Two small incisions are always made to obtain surgical access using two double-balloon secured trocars (D-B* - double-balloon secured trocars). The first one is used for the laparoscopic camera and is connected to a closed aerosol waste system (CAWS*). The second one connected to the CO2 insufflator is for the micropump nebulizer used for delivering chemotherapy under pressure via a high-pressure line.
Eight weeks after the PIPAC procedure, an exploratory laparotomy was performed. This time period has been suggested in the literature as the optimal time period between the next surgical intervention and each PIPAC surgery[20]. Macroscopically, a 3-cm tumor was palpated in the gastric body, infiltrating the gastric serosa, and no diffuse peritoneal metastasis were found anymore during a detailed standard surgical intraoperative PC lesion assessment, so complete cytoreduction (CC-0 according to Sugarbaker) appeared feasible. Therefore, curative gastrectomy, D2 lymphadenectomy and atypical liver resection were performed (Figure 4). Histopathologic evaluation demonstrated a poorly differentiated carcinoma of the stomach, which was ypT3N2 (4/40) and high grade (G3) (Figure 5). The liver metastasis had a diameter of 2.5 cm. All the resection margins were tumor-free, so the procedure was considered (potentially) curative. The postoperative course was uneventful. Four months after surgery, the patient was completely recovered and had returned to her daily activities (ECOG = 1; BMI = 20.23). Postoperative adjuvant chemotherapy with Xeloda was recommended. Narrow follow-up examinations (abdominal CT scan) will be performed.
Figure 4.
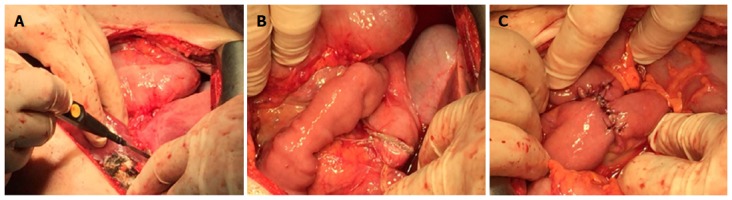
Palliative open D2 gastrectomy combined with liver metastasectomy. A: Liver metastasectomy procedure involving removal of the metastatic lesion combined with parenchyma coagulation; B: Open gastrectomy procedure showing the staple line after resection; C: Creation of a Roux-en-Y anastomosis (RNY) using sutures.
Figure 5.
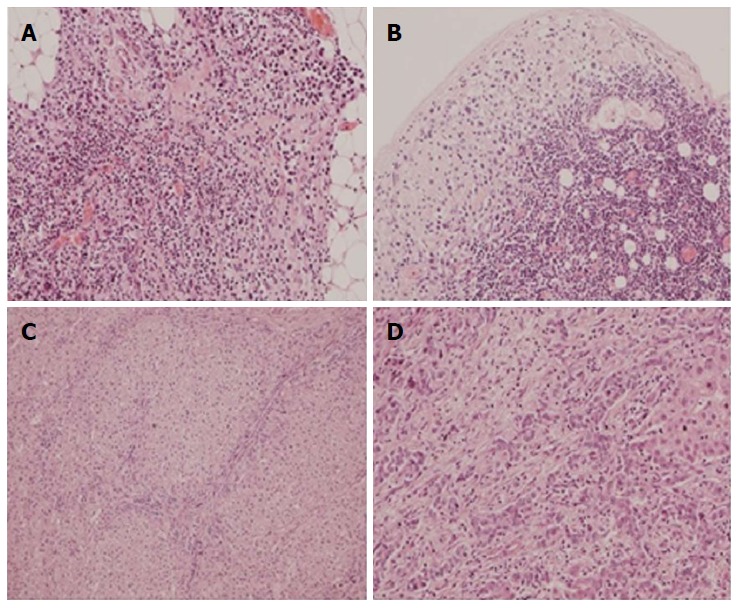
Histopathologic evaluation performed after open D2 gastrectomy combined with liver metastasectomy. A: Tumor microfocus infiltrations in the peritoneal adipose tissue in the vicinity of the distal surgical margin (obj. 20 ×, HE); B: Metastatic foci in the subcapsular region of the lymph node (obj. 20 ×, HE); C, D: Liver metastasis of gastric carcinoma (obj. 10 ×, 20 ×, HE).
DISCUSSION
The diagnosis of a Krukenberg tumor of gastric cancer origin is associated with a poorer prognosis than other types of primary origin KTs and is significantly more clinically problematic[21]. Several papers focus on different treatment options in patients with KT and mainly report on two specific issues: (1) The role of gastrectomy and metastasectomy under different clinical conditions; and (2) the assessment of the effectiveness and superiority of surgical and chemotherapy interventions[22,23]. The main problem in such descriptions is related to the fact that in large number of papers, the analyzed material concerned data only until the time of KT diagnosis[24].
In this paper, we present the case of 49-year-old PC patient with a high PCI index who was diagnosed primarily with KT due to an intrapathology assay in which bilateral hysterectomy combined with removal of the uterine appendages, omentectomy, appendectomy and pelvic lymphadenectomy was performed. The optimal treatment in such cases has still not been fully described in schematic recommendations and guidelines regarding the role of gastrectomy[25]. In recent and past literature, the beneficial outcomes of palliative gastrectomy have been presented[26]. Thus, because of the young age and good performance status of our patient along with the nonacceptance of standard intravenous forms of therapies with a parallel allowance for an intraperitoneal form of drug delivery, we elected the aforementioned treatment protocol consisting of “neoadjuvant” PIPAC followed by D2 gastrectomy. During our literature review we found information about some cases in which cytoreductive surgeries were performed in combination with hyperthermic intraperitoneal chemotherapy (CRS+HIPEC) procedures with good clinical effects that improved the survival of GC patients[27-29]. In our case, such an intervention was not possible due to the very aggressive CRS surgery and the patient’s disqualification by medical oncologists. We also found interesting data about a novel and promising intraoperative drug delivery technique - pressurized intraperitoneal aerosol chemotherapy - that has also been used as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in different clinical cases[30]. In the recent and past literature, the effectiveness of repetitive PIPAC has also been documented for irresectable PC originating from pancreatic or ovarian cancer with a median histologically proven regression rate of up to 50%[20,30-32]. In this case, our MDT recommended PIPAC as a bridge (sandwich) therapy before a possible more definitive CRS+HIPEC procedure with palliative D2 gastrectomy without other cytoreduction and final qualification for standard chemotherapy protocols, according to the current international guidelines, until these therapies are personally acceptable to the patient.
During the clinical work-up, we encountered another problematic clinical issue regarding the patients metastatic lesion in the liver, which would normally render a patient ineligible for PIPAC treatment[33]. Fortunately, the metastatic lesion in our patient was located superficially, and after PIPAC was performed, no complications were reported.
In our opinion, in the future, additional clinical studies should be performed in multiple centers to confirm the usefulness of PIPAC as a rescue or neoadjuvant, supportive form of therapy in a very select group of patients. This case might contribute to future confirmation of the usefulness of PIPAC as a rescue or neoadjuvant, supportive form of therapy in a very select group of patients. This clinical development might be particularly important for patients with a KT presentation of gastric cancer who have been recently qualified to undergo classic chemotherapy or standard oncologic surgical procedures.
ARTICLE HIGHLIGHTS
Case characteristics
A 49-year-old female patient with reduced general condition and nutritional status (low BMI and weight loss in the nutritional anamnesis) was admitted after bilateral hysterectomy with a diagnosis of diffuse peritoneal carcinomatosis and several co-morbidities.
Clinical diagnosis
The final clinical diagnosis was made by upper gastrointestinal (GI) endoscopy combined with a pathological assay that showed a mucin-positive, poorly differentiated adenocarcinoma located in the gastric antral mucosa.
Differential diagnosis
The differential diagnosis included severe peritoneal carcinomatosis and primary origin cancer with a particular emphasis on ovarian cancer.
Laboratory diagnosis
Despite the patient’s reduced general condition and nutritional status, all of the performed laboratory tests were within normal limits.
Imaging diagnosis
The CT scan performed during hospitalization in our department showed an additional superficially located metastasis in liver segment 5.
Pathological diagnosis
In this case, staining revealed CK7(+), CK20(-), CDX2(+) and CA125(-) status, suggesting a primary tumor originating from the upper GI- tract. A postoperative upper GI endoscopy showed a mucin-positive, poorly differentiated adenocarcinoma located in the gastric antral mucosa.
Treatment
The PIPAC procedure was based on the administration of a solution of low-dose cisplatin (7.5 mg/m2 BSA) and doxorubicin (1.5 mg/m2) BSA diluted in 200 mL of saline solution aerosolized at a pressure of 12 mmHg and a temperature of 37 °C into the abdomen using a CE-certified nebulizer as neoadjuvant therapy before palliative D2 gastrectomy combined with liver metastasectomy.
Related reports
Very few cases of spontaneous regression of an intra-abdominal inflammatory myofibroblastic tumor have been reported in the literature. The clinical and pathological characteristics of inflammatory myofibroblastic tumors remain unclear, and the treatment is controversial.
Term explanation
The acronym PIPAC describes pressurized intraperitoneal aerosol chemotherapy (PIPAC).
Experiences and lessons
This case might contribute to future confirmation of the usefulness of PIPAC as a rescue or neoadjuvant, supportive form of therapy in a very select group of patients. This clinical development might be particularly important for patients with a KT presentation of gastric cancer who have been recently qualified to undergo classic chemotherapy or standard oncologic surgical procedures.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Informed consent statement: Written informed consent from patient involved in this study was received prior to study inclusion. All details that might disclose the identity of the subjects under study were omitted or anonymized.
Conflict-of-interest statement: Authors declare no conflict of interest.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Peer-review started: January 31, 2018
First decision: February 24, 2018
Article in press: March 31, 2018
P- Reviewer: Huang CM, Pellicano R, Sugimura H, Tarnawski AS S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
Contributor Information
Maciej Nowacki, Department of Surgical Oncology, Ludwik Rydygier’s Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, Oncology Centre-Prof. Franciszek Łukaszczyk Memorial Hospital in Bydgoszcz, Bydgoszcz 85-796, Poland. maciej.nowacki@cm.umk.pl.
Dariusz Grzanka, Department of Clinical Pathomorphology, Ludwik Rydygier’s Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, Bydgoszcz 85-094, Poland.
Wojciech Zegarski, Department of Surgical Oncology, Ludwik Rydygier’s Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, Oncology Centre-Prof. Franciszek Łukaszczyk Memorial Hospital in Bydgoszcz, Bydgoszcz 85-796, Poland.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 3.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 5.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Vecchia C, Conte P. Cancer Control in Central and Eastern Europe. Oncologist. 2016;21:1161–1162. doi: 10.1634/theoncologist.2016-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sierra MS, Cueva P, Bravo LE, Forman D. Stomach cancer burden in Central and South America. Cancer Epidemiol. 2016;44 Suppl 1:S62–S73. doi: 10.1016/j.canep.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 9.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosseini SN, Mousavinasab SN, Moghimi MH, Fallah R. Delay in diagnosis and treatment of gastric cancer: from the beginning of symptoms to surgery--an Iranian study. Turk J Gastroenterol. 2007;18:77–81. [PubMed] [Google Scholar]
- 11.Maconi G, Manes G, Porro GB. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol. 2008;14:1149–1155. doi: 10.3748/wjg.14.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matar HE, Elmetwally AS, Salu I, Borgstein R, Oluwajobi O. Krukenberg tumour arising from adenocarcinoma of the gastro-oesophageal junction in a 28-year-old female presenting as lower abdominal swelling mimicking an inguinal hernia. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.12.2010.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubeček O, Laco J, Špaček J, Petera J, Kopecký J, Kubečková A, Filip S. The pathogenesis, diagnosis, and management of metastatic tumors to the ovary: a comprehensive review. Clin Exp Metastasis. 2017;34:295–307. doi: 10.1007/s10585-017-9856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah B, Tang W, Karn S. An Up-to-Date Understanding of the “Krukenberg Tumor” Mechanism. ARSci. 2016;4:31–36. [Google Scholar]
- 15.Jeung YJ, Ok HJ, Kim WG, Kim SH, Lee TH. Krukenberg tumors of gastric origin versus colorectal origin. Obstet Gynecol Sci. 2015;58:32–39. doi: 10.5468/ogs.2015.58.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu KY, Gao H, Lian ZJ, Ding L, Li M, Gu J. Clinical analysis of Krukenberg tumours in patients with colorectal cancer-a review of 57 cases. World J Surg Oncol. 2017;15:25. doi: 10.1186/s12957-016-1087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y, Hiramatsu A, Koyama T, Oyama Y, Tanaka A, Honma K. A Krukenberg Tumor from an Occult Intramucosal Gastric Carcinoma Identified during an Autopsy. Case Rep Oncol Med. 2014;2014:797429. doi: 10.1155/2014/797429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu W, Yuan L, Liu X, Guo SW. Identification of prognostic factors for Krukenberg tumor. GMIT. 2013;2:52–56. [Google Scholar]
- 19.Hübner M, Grass F, Teixeira-Farinha H, Pache B, Mathevet P, Demartines N. Pressurized IntraPeritoneal Aerosol Chemotherapy - Practical aspects. Eur J Surg Oncol. 2017;43:1102–1109. doi: 10.1016/j.ejso.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Alyami M, Gagniere J, Sgarbura O, Cabelguenne D, Villeneuve L, Pezet D, Quenet F, Glehen O, Bakrin N, Passot G. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43:2178–2183. doi: 10.1016/j.ejso.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Jiang R, Tang J, Cheng X, Zang RY. Surgical treatment for patients with different origins of Krukenberg tumors: outcomes and prognostic factors. Eur J Surg Oncol. 2009;35:92–97. doi: 10.1016/j.ejso.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Lu LC, Shao YY, Hsu CH, Hsu C, Cheng WF, Lin YL, Cheng AL, Yeh KH. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res. 2012;32:3397–3401. [PubMed] [Google Scholar]
- 23.Cho JH, Lim JY, Choi AR, Choi SM, Kim JW, Choi SH, Cho JY. Comparison of Surgery Plus Chemotherapy and Palliative Chemotherapy Alone for Advanced Gastric Cancer with Krukenberg Tumor. Cancer Res Treat. 2015;47:697–705. doi: 10.4143/crt.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGill F, Ritter DB, Rickard C, Kaleya RN, Wadler S, Greston WM. Management of Krukenberg tumors: an 11-year experience and review of the literature. Prim Care Update Ob Gyns. 1998;5:157–158. doi: 10.1016/s1068-607x(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 25.Khosla M, Imran A, Fidias P. Diffuse gastric cancer with Krukenberg tumor: A case report. Int J Case Rep Images. 2016;7:733–737. [Google Scholar]
- 26.Hsu JT, Liao JA, Chuang HC, Chen TD, Chen TH, Kuo CJ, Lin CJ, Chou WC, Yeh TS, Jan YY. Palliative gastrectomy is beneficial in selected cases of metastatic gastric cancer. BMC Palliat Care. 2017;16:19. doi: 10.1186/s12904-017-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu XJ, Yuan P, Li ZY, Bu ZD, Zhang LH, Wu AW, Zong XL, Li SX, Shan F, Ji X, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves the survival of gastric cancer patients with ovarian metastasis and peritoneal dissemination. Tumour Biol. 2013;34:463–469. doi: 10.1007/s13277-012-0571-4. [DOI] [PubMed] [Google Scholar]
- 28.Li-Yung C, Chun-Yu F, Huai-En L, De-Chua C. Treatment of Krukenberg tumor with hyperthermic intraperitoneal chemotherapy: A report of three cases. JMS-NDMC. 2016;36:197–201. [Google Scholar]
- 29.Nowacki M, Wisniewski M, Werengowska-Ciecwierz K, Roszek K, Czarnecka J, Łakomska I, Kloskowski T, Tyloch D, Debski R, Pietkun K, et al. Nanovehicles as a novel target strategy for hyperthermic intraperitoneal chemotherapy: a multidisciplinary study of peritoneal carcinomatosis. Oncotarget. 2015;6:22776–22798. doi: 10.18632/oncotarget.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2016;14:253. doi: 10.1186/s12957-016-1008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graversen M, Detlefsen S, Bjerregaard JK, Pfeiffer P, Mortensen MB. Peritoneal metastasis from pancreatic cancer treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) Clin Exp Metastasis. 2017;34:309–314. doi: 10.1007/s10585-017-9849-7. [DOI] [PubMed] [Google Scholar]
- 32.Khosrawipour T, Khosrawipour V, Giger-Pabst U. Pressurized Intra Peritoneal Aerosol Chemotherapy in patients suffering from peritoneal carcinomatosis of pancreatic adenocarcinoma. PLoS One. 2017;12:e0186709. doi: 10.1371/journal.pone.0186709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilal Z, Rezniczek GA, Klenke R, Dogan A, Tempfer CB. Nutritional status, cachexia, and anorexia in women with peritoneal metastasis and intraperitoneal chemotherapy: a longitudinal analysis. J Gynecol Oncol. 2017;28:e80. doi: 10.3802/jgo.2017.28.e80. [DOI] [PMC free article] [PubMed] [Google Scholar]


