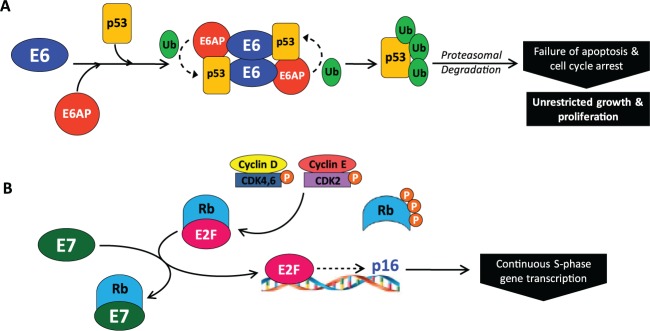
Figure 2.
E6 and E7 induce replication by blocking the function of cell cycle regulators. (A) The human papillomavirus (HPV) oncoprotein E6 recruits the cellular E3 ubiquitin ligase, E6-associated protein (E6AP) (Talis et al. 1998), and binds TP53, leading to TP53 polyubiquitination and subsequent degradation by the 26S proteasome. The destruction of TP53 results in failure of cell cycle arrest and apoptosis, contributing to unrestricted host cell growth and proliferation. (B) In normal cells, the cell cycle regulator RB binds the transcription factor E2F, preventing cell cycle progression. When cell growth signaling occurs, expression of cyclin D1 is initiated. Cyclin D1 activates cyclin-dependent kinase (CDK) 4/6, leading to monophosphorylation of RB. CDK2 is then activated by cyclin E and further phosphorylates RB, releasing E2F and initiating transcription of cell cycle entry genes. E2F also activates transcription of p16INK4a (CDKN2A, an inhibitor of cyclin-dependent kinase 4 and the off signal for RB phosphorylation), shutting off RB phosphorylation. Ubiquitous phosphatase activity dephosphorylates RB, which resequesters E2F and stops cell cycle entry. In the presence of HPV, E7 binds to the pocket of RB, disrupting the interaction with E2F. When E2F is liberated, it leads to continual transcription of S-phase genes, driven by other cell cycle cyclin-CDK complexes. p16INK4a is also inappropriately transcribed and expressed, making it a useful surrogate histological marker of HPV infection. The binding of E7 to RB leads to continuous cell cycle entry, progression, and cellular proliferation.