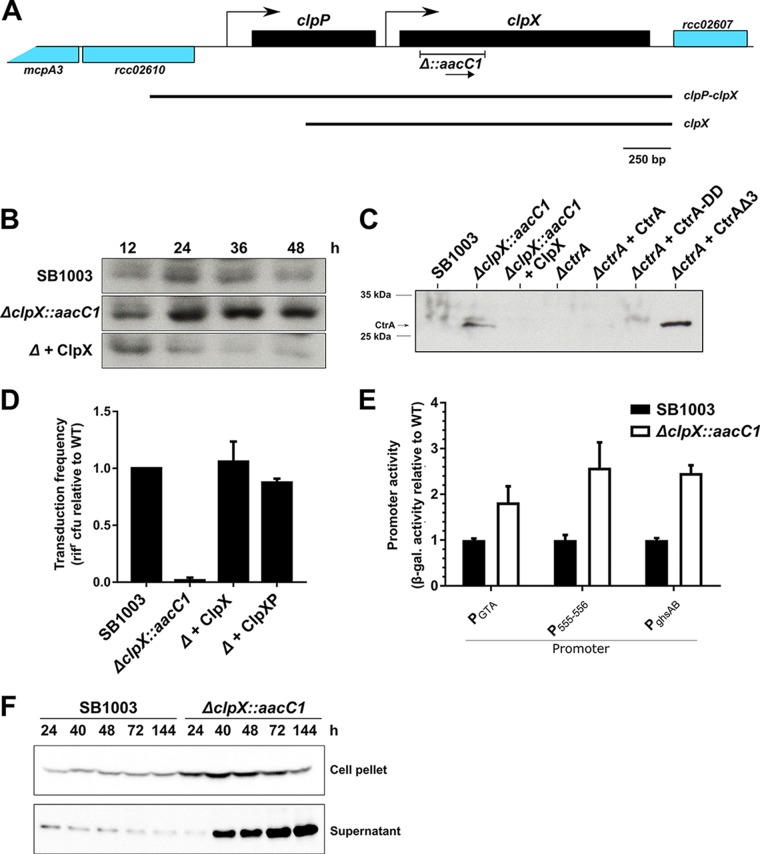
FIG 3.
ClpX(P) regulates CtrA levels and is required for RcGTA-mediated gene transfer. (A) Genetic context of clpX and clpP in R. capsulatus (see Fig. S2B in the supplemental material for the genetic context in other bacteria). Predicted promoters (bent arrows), the fragment replaced by aacC1 to create the ΔclpX::aacC1 mutant, and fragments included in the complementation plasmids pABW710 and pLK718 are indicated. The transcriptional direction of aacC1 is indicated by an arrow. The figure is drawn approximately to scale. (B and C) Immunoblots of CtrA levels in cells. The membranes were probed using C. crescentus CtrA antiserum. (B) A time course experiment of CtrA in WT strain SB1003, the SB1003 ΔclpX::aacC1 mutant, and the mutant containing clpX in trans on pABW710. (C) CtrA levels in SB1003 and the derived ΔclpX::aacC1 and ΔctrA mutants encoding ClpX (pABW710), WT CtrA (pLK754), CtrA-DD (pLK755), or CtrAΔ3 (pLK756) in trans, as indicated. (D) RcGTA-mediated gene transfer frequency of the culture supernatant of SB1003, the SB1003 ΔclpX::aacC1 mutant (Δ), and the mutant containing clpX (pABW710) or clpP-clpX (pLK718) in trans. (E) Activities of three RcGTA promoters in SB1003 and SB1003 ΔclpX::aacC1 mutant cells. The β-galactosidase activity of cells containing the RcGTA head-tail cluster reporter p601-g65 (PGTA), the endolysin-holin reporter pXCA-555 (P555-556), and the ghsAB head spike reporter pXCA-ghsA (PghsAB) is shown. (F) Production and release of cleaved RcGTA capsid protein by SB1003 and SB1003 ΔclpX::aacC1. An immunoblot of the supernatant (extracellular) and cell pellet (intracellular) fractions is shown. The membranes were probed using RcGTA capsid antiserum. Cells were cultured for an equivalent number of cell divisions (C to E) or harvested after the indicated times (in hours) of incubation after inoculation (B and F). Bars represent means, and error bars represent the standard deviations for two biological replicates (D and E).