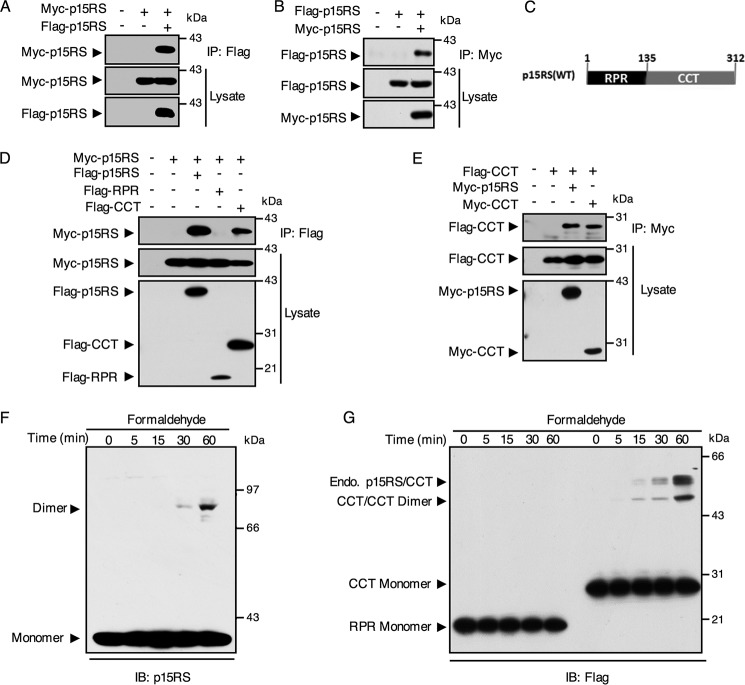
Figure 1.
The CCT domain of p15RS mediates its dimerization in vitro. A and B, homologous interaction of p15RS in vitro. Lysates of HEK293T cells transfected with Myc-tagged and FLAG-tagged p15RS were immunoprecipitated by anti-FLAG antibody (A) or anti-Myc antibody (B) and subjected to Western blotting with the indicated antibodies. C, graphic representation of p15RS protein structure: RPR domain from amino acids 1 to 135 and CCT domain from amino acids 136 to 312. D, CCT domain is responsible for the dimerization of p15RS. FLAG-tagged full-length p15RS, RPR, or CCT domains were co-expressed with Myc-tagged full-length p15RS in HEK293T cells. Cell lysates were incubated with an anti-FLAG antibody for the IP assay. E, the CCT domain of p15RS per se dimerizes. Myc-tagged full-length (Myc-p15RS) or CCT domain of p15RS (Myc-CCT) were co-expressed in HEK293T cells with the FLAG-tagged CCT domain of p15RS (Flag-CCT). An anti-Myc antibody was used for immunoprecipitation. F, p15RS forms a homodimer. HEK293T cells transiently overexpressing Flag-p15RS were cross-linked by 1% formaldehyde for the indicated times at room temperature 24 h after transfection. An anti-p15RS antibody was used to detect the 39-kDa monomer and the 78-kDa dimer of Myc-p15RS. G, the CCT domain of p15RS determines dimerization, whereas the RPR domain stays as monomer. HEK293T cells transfected with FLAG-tagged RPR or CCT domains of p15RS were subjected to cross-linking with 1% formaldehyde for the indicated times. The monomers and dimers were revealed by Western blotting using an anti-FLAG antibody. Note that dimers of endogenous p15RS with the CCT domain are also marked.