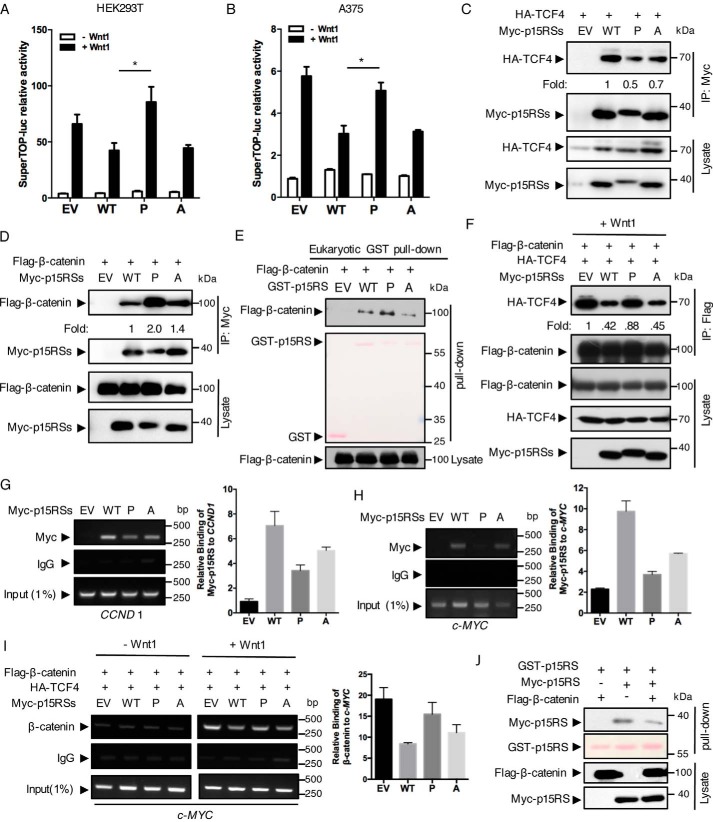
Figure 3.
The dimeric ability of p15RS is required for its inhibitory role on Wnt signaling. A and B, dimerization of p15RS participates in the inhibition of Wnt1-stimulated transcriptional activity. Luciferase assays were performed using HEK293T (A) or A375 (B) cells with transient expression of Myc-tagged p15RS (referred hereafter to as WT), p15RSL248P/L255P (referred hereafter to as P), or Flag-p15RSL248A/L255A (referred hereafter to as A) together with a SuperTop-luciferase reporter and pRL-TK (as an internal control). EV indicates empty vector as a control. Wnt1 expression was generated by transfection of a Wnt1 plasmid. Relative luciferase activities were normalized with the internal control. Results are presented from three independent experiments, and data are represented as mean ± S.D. (n = 3). Asterisk indicates a statistically significant difference. *, p < 0.05. C, p15RSL248P/L255P interacts with TCF4 with a decreased affinity. Myc-tagged p15RS, p15RSL248P/L255P, or p15RSL248A/L255A were co-expressed with HA-TCF4 in HEK293T cells. Cell lysates were incubated with an anti-Myc antibody and subjected to Western blotting by an anti-HA antibody. Relative binding affinity was represented as fold-change based on the level of the HA-TCF4 and Myc-p15RS. D and E, decreased dimerization leads to tighter bond between p15RS and β-catenin. Myc-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A were co-expressed with FLAG-β-catenin in HEK293T cells. Cell lysates were incubated with an anti-Myc antibody and subjected to Western blotting by an anti-FLAG antibody. Cell lysates expressing FLAG-β-catenin were incubated with eukaryotic purified GST-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A proteins, together with GST beads, and then subjected to Western blotting by an anti-FLAG antibody (E). F, diminished dimerization of p15RS enhances the interaction of β-catenin and TCF4. HA-TCF4 and FLAG-β-catenin were co-expressed with Myc-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A in HEK293T cells. The cell lysates were subjected to an IP experiment with an anti-FLAG antibody and examined by Western blotting using the indicated antibodies. G and H, dimerization of p15RS is critical for its occupancy on the TBS of Wnt-targeted gene promoters. HEK293T cells were transfected with Myc-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A and cross-linked to perform a ChIP assay using an anti-Myc antibody. The co-immunoprecipitated DNA was examined by PCRs or quantitative PCRs using specific primers for TBS of CCND 1 (G) or c-MYC (H). I, dimerization of p15RS is crucial for its blockage of the TCF4·β-catenin complex to occupy on the TBS of c-MYC. HA-TCF4 and FLAG-β-catenin were co-expressed with Myc-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A in HEK293T cells. Cells were cross-linked to perform a ChIP assay using an anti-β-catenin antibody. Primers specifically recognizing the TBS of c-MYC were used to detect and quantify the precipitated DNAs. J, β-catenin weakens the dimeric interaction of p15RS. HEK293T cells transfected with GST-p15RS, Myc-p15RS, or FLAG-β-catenin were precipitated by GST beads and the dimerized p15RS was examined by Western blotting using an anti-Myc antibody.