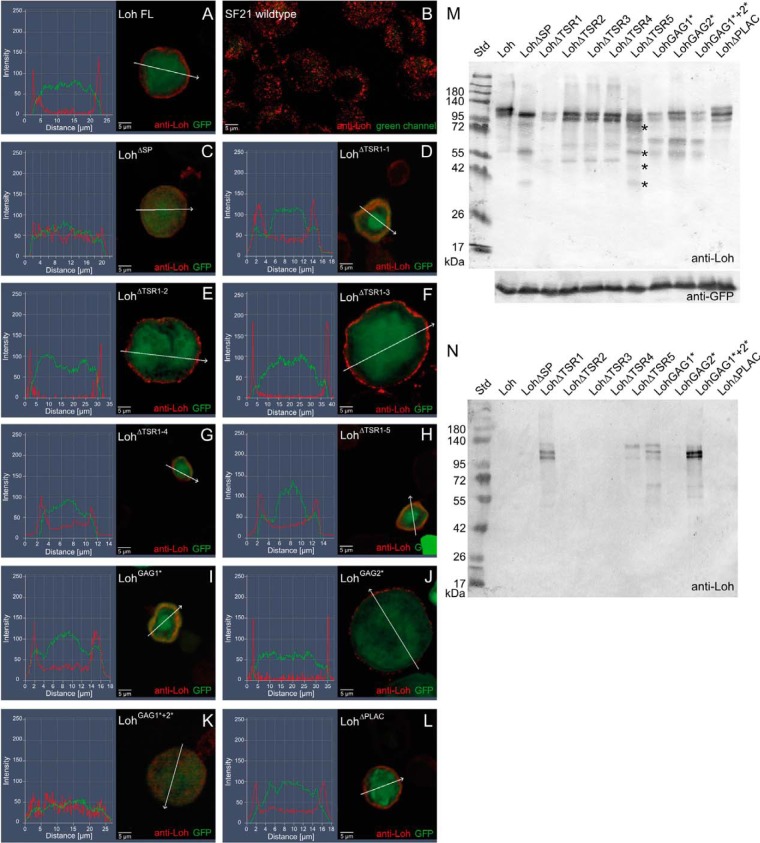
Figure 2.
Expression and localization of mutated Loh constructs in Sf21 cells. Sf21 cells were transfected with WT and individually mutated Loh constructs. Successful transfection was monitored by simultaneous expression of cytoplasmic GFP (green channel). WT as well as mutated Loh were visualized by anti-Loh antibody staining (red channel). Intensity profiling of stained cells was used to determine the subcellular localization of individual Loh constructs. The respective regions of evaluation are marked (arrows in A–L). Full-length Loh (A) is secreted and accumulates at the surface of the cell. Anti-Loh staining of untransfected cells results in a spotted distribution of low-intensity signals (overexposed to visualize the shape of the cells) (B). Loh lacking the signal peptide is not secreted and retained in the cytoplasm (C). The constructs LohΔTSR1-1 and LohGAG1* show no distinct surface location but accumulate in a patchy manner close to the plasma membrane (D and I). A similar behavior is observed for LohGAG1*+2*, yet with a broader distribution in the cytoplasm (K). Individual mutations in the remaining thrombospondin type I repeat domains (LohΔTSR1-2, LohΔTSR1-3, LohΔTSR1-4, and LohΔTSR1-5), as well as lack of the PLAC domain (LohΔPLAC) or a mutation in the second speculative GAG binding site (LohGAG2*), do not affect secretion or anchoring (E–H, J, and L). M, Western blotting of construct-specific cell lysates confirms expression and adequate molecular mass of all Loh constructs. Asterisks indicate putative degradation products that are unique to LohΔTSR1-5 and do not appear in the case of any other TSR1 deletion construct. Co-transfected GFP was used as a loading control. N, Western blotting of construct-specific cell culture media confirms the presence of LohΔTSR1-1, LohΔTSR1-5, LohGAG1*, and LohGAG1*+2*, indicating proper secretion but impaired binding of the respective constructs to the ECM. The depicted blots are representative of three individual biological replicates.