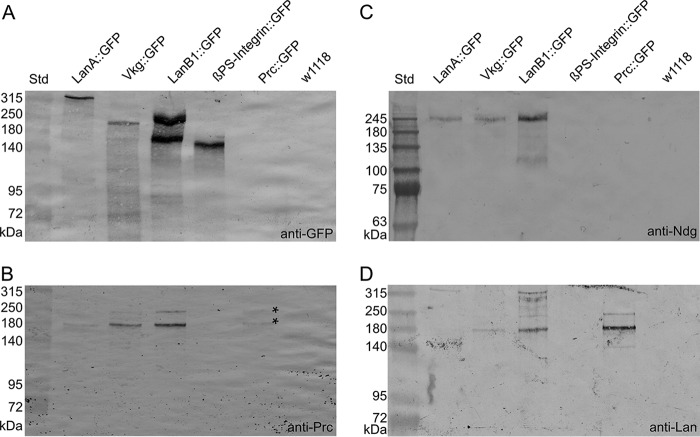
Figure 7.
Pericardin co-immunoprecipitates with distinct ECM components. GFP-tagged ECM proteins (laminin A, LanA::GFP; laminin B1, LanB1::GFP; Viking, Vkg::GFP; βPS-integrin, βPS-integrin::GFP; Pericardin, Pericardin::GFP) were purified from third instar larvae and subjected to Western blot analysis. Anti-GFP antibodies were applied to estimate the protein-specific purification efficiency (A). Anti-Pericardin (Prc; B), anti-nidogen (Ndg; C), and anti-laminin (Lan; D) antibodies were used to assess co-immunoprecipitation of the individual ECM components with the purified GFP fusion proteins. The rather weak detection of endogenous Pericardin in the Pericardin::GFP sample is indicated (B; asterisks). The observation that nidogen migrates significantly higher than expected (predicted molecular mass, 149.1 kDa) presumably reflects extensive posttranslational modification of the protein. Similar mass shifts, predominantly caused by N- and O-glycosylation, have been reported for nidogens from other species (4). The depicted blots are representative of two individual biological replicates.