Figure 1.
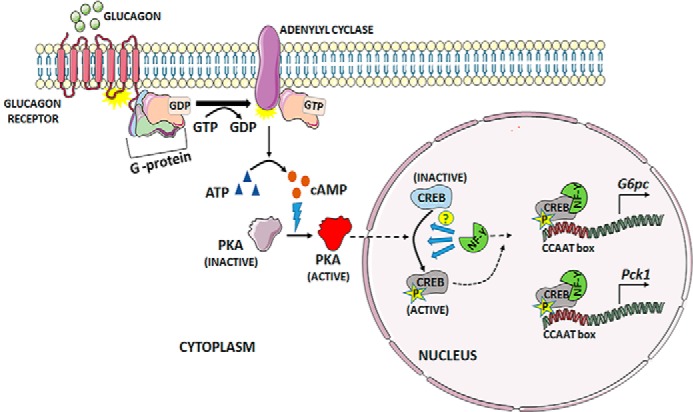
Role of NF-Y in glucagon-mediated activation of the gluconeogenic genes G6pc and Pck1 through the cAMP–CREB axis. Binding of glucagon to its receptor in hepatocytes triggers the guanylyl nucleotide exchange, leading to dissociation of the activated, GTP-bound Gα subunit from the heterotrimeric G-protein complex. Binding of the active Gα subunit to adenylyl cyclase triggers the conversion of ATP to the cyclic AMP. This, in turn, activates protein kinase A (PKA), which then translocates to the nucleus and phosphorylates CREB to make it transcriptionally active. Zhang et al. (7) show that NF-Y is key in mediating this phosphorylation step, although the exact mechanism is yet to be explored. The authors also show that activated CREB binds to NF-Y, ultimately up-regulating expression of G6pc and Pck1 by binding to the CCAAT regulatory region (7).
