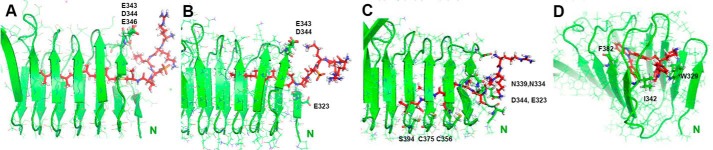
Figure 7.
MD simulation and models for lipidated and unmodified peptides and for deltarasin inhibitor. Interactions of RKKSSC-Rap1b-GerGer (A) and -Far (B) prenylated peptides with the N-terminal opening of C-CAP after 5 ns of all-atom dynamics simulation are shown. Both peptides are phosphorylated on Ser179. Although the GG lipid tail is maintained along nearly the entire length of the tunnel, the Far-modified peptide substantially slides out of the tunnel, consistent with weaker binding experimentally (the Far group is initially ∼10 Å shorter than GerGer). Several salt bridges exist between peptide Lys/Arg residues and C-CAP Asp/Glu residues side chains at the N-terminal mouth. In the case of unprocessed peptide and deltarasin inhibitor, initial structures after minimization are shown (C and D). This peptide may be stabilized by the nonpolar side chains in the tunnel (labeled in C), whereas the carbon rings of the inhibitor deltarasin may be stabilized by Phe/Trp and Ile upon initial insertion into N-terminal C-CAP as shown (D). Note that D shows the CAP1 tunnel entrance from the N-terminal side, different from the side-on view of the other panels (A–C).