Daily PrEP dosing recommendations led to a greater likelihood of protective drug concentrations in the Harlem cohort of men and transgender women who have sex with men, while daily and nondaily regimens led to comparably favorable outcomes in Bangkok.
Keywords: HIV, prevention, preexposure prophylaxis, men who have sex with men, transgender women
Abstract
Background
Nondaily dosing of oral preexposure prophylaxis (PrEP) may provide equivalent coverage of sex events compared with daily dosing.
Methods
At-risk men and transgender women who have sex with men were randomly assigned to 1 of 3 dosing regimens: 1 tablet daily, 1 tablet twice weekly with a postsex dose (time-driven), or 1 tablet before and after sex (event-driven), and were followed for coverage of sex events with pre- and postsex dosing measured by weekly self-report, drug concentrations, and electronic drug monitoring.
Results
From July 2012 to May 2014, 357 participants were randomized. In Bangkok, the coverage of sex events was 85% for the daily arm compared with 84% for the time-driven arm (P = .79) and 74% for the event-driven arm (P = .02). In Harlem, coverage was 66%, 47% (P = .01), and 52% (P = .01) for these groups. In Bangkok, PrEP medication concentrations in blood were consistent with use of ≥2 tablets per week in >95% of visits when sex was reported in the prior week, while in Harlem, such medication concentrations occurred in 48.5% in the daily arm, 30.9% in the time-driven arm, and 16.7% in the event-driven arm (P < .0001). Creatinine elevations were more common in the daily arm (P = .050), although they were not dose limiting.
Conclusions
Daily dosing recommendations increased coverage and protective drug concentrations in the Harlem cohort, while daily and nondaily regimens led to comparably favorable outcomes in Bangkok, where participants had higher levels of education and employment.
Clinical Trials Registration
Daily dosing of preexposure prophylaxis (PrEP) is recommended by the US Food and Drug Administration and the Centers for Disease Control and Prevention [1], whereas the European AIDS Clinical Society recommends either daily dosing or dosing before and after sex [2]. The Ipergay trial demonstrated effectiveness of “on demand” dosing, involving a double emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) dose 2–24 hours before sex and an additional FTC/TDF dose on each of the 2 days following sex [3], confirming findings from animal models [4]. The Iniciativa Profilaxis Pre-exposición (iPrEx) open-label extension found no new infections among men with drug concentrations indicating the use of ≥4 tablets of FTC/TDF per week as well as an 81% reduction in human immunodeficiency virus (HIV) incidence associated with average use of 2–3 tablets per week [5].
Adherence to event-driven dosing recommendations has been mixed. Such dosing was associated with lower PrEP adherence in 2 small blinded and placebo-controlled PrEP trials conducted in Africa [6, 7]. The use of blinding and a placebo may have undermined PrEP use in these trials. Furthermore, different measurements provided highly divergent estimates of adherence, which highlighted the methodological challenges arising when assessing event-driven PrEP dosing.
The HIV Prevention Trials Network (HPTN) study 067, the Alternative Dosing to Augment PrEP Pill Taking (ADAPT) study, randomly assigned participants to daily or nondaily dosing of open-label oral FTC/TDF. The aim was to assess the likelihood that sex events would be covered by pre- and postexposure antiretroviral dosing with daily or nondaily regimens that were effective in animal models [4] and the likelihood of achieving drug concentrations that provide substantial reductions in HIV incidence among men and transgender women who have sex with men [5].
METHODS
Study Design
The HPTN 067 study was a phase 2, randomized, open-label, pharmacokinetic, and behavioral equivalence study of daily vs nondaily oral FTC/TDF PrEP. The study enrolled men and transgender women who have sex with men at a community clinic and clinical research site in Bangkok, Thailand, and a clinical research site in Harlem in New York City. Women were enrolled at a study site in Cape Town, South Africa, as reported separately [8]. Ethics committee approvals were obtained from all applicable authorities (see Supplementary Materials). The protocol was registered at ClinicalTrials.gov (identifier NCT01327651; https://www.hptn.org/research/studies/82).
Participants
Participants were eligible based on the following criteria: male sex assigned at birth, HIV-antibody negative, at least 18 years old, normal renal function (estimated creatinine clearance >70 mL/minute), hepatitis B surface antigen negative, literate in English or Thai, able to provide written informed consent, and reported anal or neovaginal sex with a man in the past 6 months, and have at least 1 of the following self-reported risk factors for HIV acquisition in the past 6 months: sex with >1 man or transgender woman; history of an acute sexually transmitted infection; sex in an exchange for money, goods, or favors; or intercourse without a condom with an HIV-infected partner or partner of unknown HIV infection status (see Supplementary Materials for eligibility related to hepatitis B immunity).
Visits and Medication Dispensation
Study visits were divided into phases for screening, directly observed dosing, self-administered dosing, and post-PrEP use. Visits were scheduled at screening; enrollment (the beginning of directly observed dosing); weeks 1, 2, 3, and 4 (the end of directly observed dosing); weeks 5 and 6 (randomization and the beginning of self-administered dosing); weeks 10, 14, 18, 22, 26, and 30 (end of self-administered dosing); and week 34. To facilitate interpretation of drug concentration results during the study, participants received once per week directly observed dosing of 1 tablet of oral FTC/TDF at enrollment and at weeks 1, 2, 3, and 4. At week 6, participants were randomized and were dispensed 30 tablets of oral FTC/TDF and provided counseling specific to their randomization group (see Supplementary Materials). Every 4 weeks during the self-administered phase, an additional 30 tablets of FTC/TDF were dispensed.
Monitoring of PrEP Use and Sex
All participants received a real-time electronic drug monitoring (EDM) device (WisePill) at enrollment. Weekly phone-based or in-person interviews at the study site (at the choice of the participant) were conducted using real-time EDM data to determine if an electronically recorded “opening” event was reflective of an ingested dose (vs curiosity opening, pocket dose, or refill of device) and for correction of date and time for doses removed and taken at a later time; after collecting dose date and time information, sexual events over the past week were documented for date and time, as well as type of sex, condom use, and partners. Interviewers were not part of the clinical care team and did not feed back information to team members and were trained in neutral interviewing [9]. The results of the EDM-guided weekly interviews formed the basis for assessing coverage of sex events with pre- and postexposure dosing and adherence (see Supplementary Materials). Participants were offered vitamin tablets during the 6-week directly observed therapy phase (to become familiar with the device). Long-term adherence to PrEP was evaluated using blood concentrations of tenofovir diphosphate (TFV-DP) in dried blood spots [5, 10] or peripheral blood mononuclear cells [11] (see Supplementary Materials). Tablet sharing was investigated by a computer-assisted self-interview conducted 12 and 24 weeks after randomization.
Primary Outcomes
The primary outcome was coverage of anal and neovaginal intercourse events with pre- and postexposure dosing of PrEP defined as at least 1 tablet reported taken within 96 hours (4 days) prior to intercourse and another tablet reported taken within 24 hours after intercourse. Drug concentrations during weeks when sex was reported should reflect use of at least 2 PrEP tablets if the sex event was covered with pre- and postsex dosing according to the regimens recommended in the protocol. This amount of oral FTC/TDF PrEP use protected nonhuman primates [4] and reduced HIV incidence in men who have sex with men (MSM) by 76% [11]. See the Supplementary Materials for definitions of outcomes.
Statistical Analysis
Quantitative measures are summarized using medians and interquartile ranges; categorical measures are summarized with proportions. In the primary analysis of coverage, the unit of analysis is the sex act (covered or not covered). A logistic regression for dependent data [12] with robust variance and clustering on participant was used to compare coverage between the daily arm and each intermittent arm (P values for a standard inequality test are given and we comment on noninferiority as needed). A similar approach (with the follow-up visit as the unit of analysis) was used to compare the prevalence of neurological and gastroenterological side effects between arms. The number of pills used and the number of pills required to achieve full adherence to the recommended regimens were compared using a log regression with offset equal to the log of the duration of follow-up and a robust variance. All analyses were intent to treat. Individuals were dropped from analyses following HIV seroconversion.
RESULTS
Study Participants
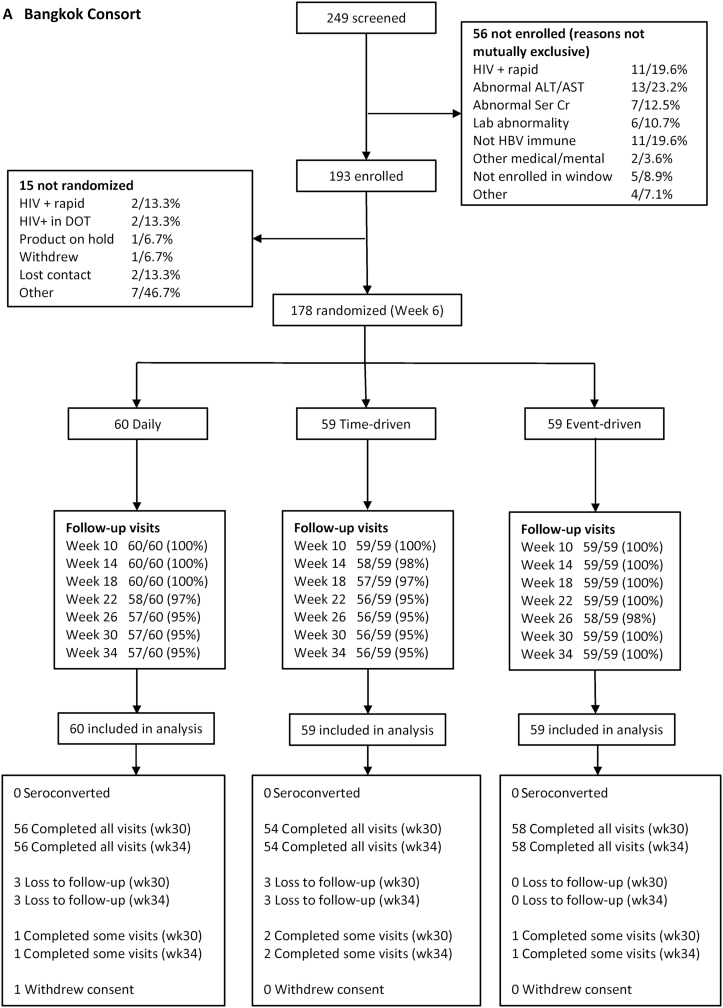
From 4 July 2012 to 6 May 2014, 608 people were screened, 431 were enrolled, and 357 (83%) were randomized and included in the analysis (Figure 1A and 1B). One randomized participant in Bangkok was excluded from the analysis, as instructed by the local institutional review board, due to a protocol deviation. Retention through the end of the self-administered dosing phase (week 30) was 97% at the Bangkok site and 83% at the Harlem site. Overall, among people randomized, 350 identified as a man, 5 identified as a transgender woman, and 2 identified as gender queer (Table 1). Participants in Bangkok were more likely than those in Harlem to have at least secondary education (P < .0001) and be fully employed (P < .0001). Among the 179 participants in Harlem, race/ethnicity was self-reported in nonexclusive categories: 126 (70%) were black, 23 (13%) were white, 5 (3%) were Asian, 5 (3%) were Native American, 44 (25%) were Hispanic, and 37 (21%) were other.
Figure 1.
Consort diagrams for the HIV Prevention Trials Network (HPTN) 067 ADAPT study in Bangkok, Thailand (A) and Harlem, New York (B). Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DOT, directly observed therapy; HBV, hepatitis B virus; HIV, human immunodeficiency virus; Ser Cr, serum creatinine.
Table 1.
Baseline Characteristics Among Participants Randomized in the HIV Prevention Trials Network (HPTN) 067 Study, Bangkok, Thailand (n = 178) and Harlem, New York (n = 179)
| Characteristic | Bangkok | Harlem | ||||
|---|---|---|---|---|---|---|
| Daily | Time-Driven | Event-Driven | Daily | Time-Driven | Event-Driven | |
| Age, y | ||||||
| 18–24 | 8 (13.3) | 12 (20.3) | 8 (13.5) | 19 (32.2) | 17 (28.3) | 17 (28.3) |
| 25–29 | 13 (21.7) | 19 (32.2) | 16 (27.1) | 13 (22.0) | 11 (18.4) | 8 (13.4) |
| 30–39 | 36 (60.0) | 23 (39.0) | 28 (47.5) | 11 (18.7) | 12 (20.0) | 14 (23.3) |
| ≥40 | 3 (5.0) | 5 (8.5) | 7 (11.9) | 16 (27.1) | 20 (33.3) | 21 (35.0) |
| Self-identified gender | ||||||
| Man | 59 (98.3) | 58 (98.3) | 59 (100) | 57 (96.6) | 59 (98.3) | 58 (96.6) |
| Transgender woman | 1 (1.7) | 1 (1.7) | 0 (0) | 2 (3.4) | 0 (0) | 1 (1.7) |
| Gender queer | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.7) | 1 (1.7) |
| Schooling | ||||||
| Less than secondary | 0 (0) | 1 (1.7) | 1 (1.7) | 16 (27.1) | 18 (30.0) | 7 (11.7) |
| Completed secondary | 1 (1.7) | 7 (11.9) | 1 (1.7) | 10 (17.0) | 19 (31.7) | 33 (55.0) |
| More than secondary | 59 (98.3) | 51 (86.4) | 57 (96.6) | 33 (55.9) | 23 (38.3) | 20 (33.3) |
| Employment | ||||||
| None | 8 (13.3) | 9 (15.2) | 3 (5.1) | 40 (67.8) | 39 (65.0) | 44 (73.3) |
| Part-time | 2 (3.3) | 5 (8.5) | 3 (5.1) | 6 (10.2) | 14 (23.3) | 10 (16.7) |
| Full-time | 50 (83.4) | 45 (76.3) | 53 (89.8) | 13 (22.0) | 7 (11.7) | 6 (10.0) |
| Sex partners (past 3 mo) | ||||||
| 0–1 | 17 (28.3) | 16 (27.1) | 10 (17.0) | 3 (5.1) | 4 (6.7) | 4 (6.7) |
| 2–4 | 19 (31.7) | 24 (40.7) | 29 (49.2) | 30 (50.8) | 21 (35.0) | 26 (43.3) |
| 5–9 | 16 (26.7) | 6 (10.2) | 11 (18.6) | 8 (13.6) | 18 (30.0) | 18 (30.0) |
| ≥10 | 8 (13.3) | 13 (22.0) | 9 (15.2) | 17 (28.8) | 15 (25.0) | 12 (20.0) |
| Missing | 0 (0) | 0 (0) | 0 (0) | 1 (1.7) | 2 (3.3) | 0 (0) |
| Anal intercourse without a condom | ||||||
| No | 38 (63.3) | 33 (55.9) | 42 (71.2) | 12 (20.3) | 20 (33.3) | 10 (16.7) |
| Yes | 22 (36.7) | 26 (44.1) | 17 (28.8) | 47 (79.7) | 40 (66.7) | 50 (83.3) |
Data are presented as No. (%).
Coverage and PrEP Use
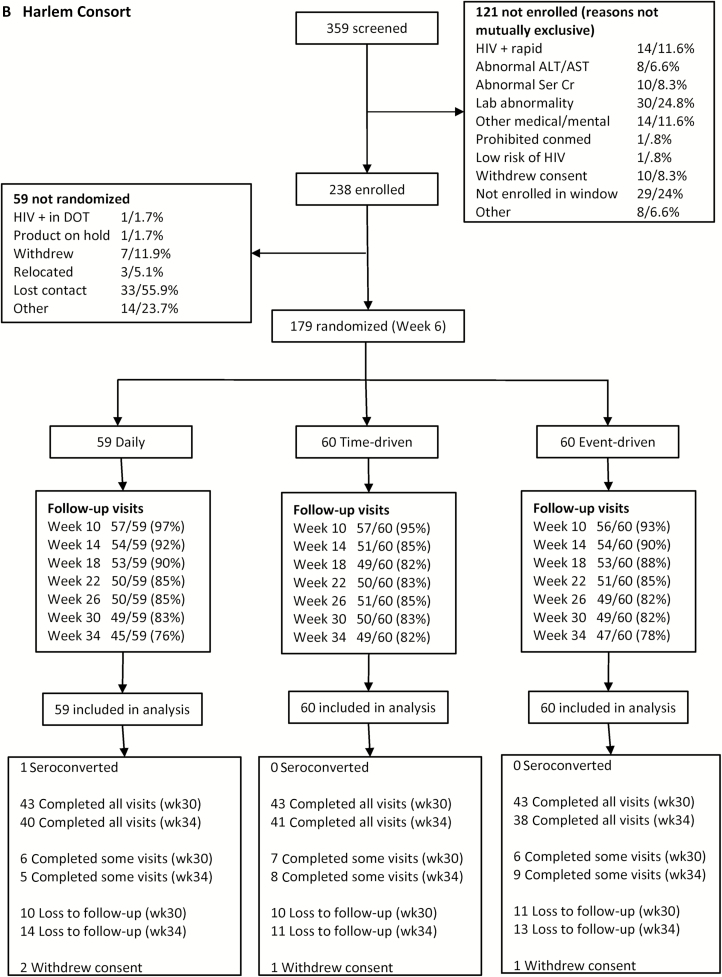
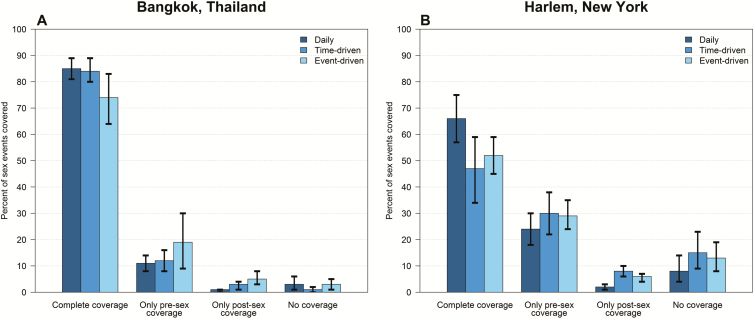
Overall, 357 people reported 7734 sex events during the 24-week self-administered phase (average 0.9 sex events per person per week). In Bangkok, coverage of sex events was 85% (1266/1485) for the daily arm compared with 84% (1129/1337) for the time-driven arm (P = .79 vs daily), and 74% (749/1018) for the event-driven arm (P = .02 vs daily) (Figure 2A). In Harlem, coverage was 66% (718/1081) for the daily arm compared with 47% (615/1311) for the time-driven arm (P = .01 vs daily) and 52% (786/1502) for the event-driven arm (P = .01 vs daily) (Figure 2B). Among people with partial coverage, the postsex dose was more commonly missed than the presex dose at both sites (Figure 2). Coverage did not change over time in any arm at either site (P = .98 for Bangkok, P = .20 for Harlem; Figure 3). The results were not different when coverage of sex events without a condom was considered.
Figure 2.
Coverage of sex events with pre- and postsex preexposure prophylaxis dosing in the HIV Prevention Trials Network (HPTN) 067 study, Bangkok, Thailand (A) and Harlem, New York (B). Error bars represent the 95% confidence interval of the estimate of coverage, based on bootstrap analysis.
Figure 3.
Coverage of sex events with pre- and postsex dosing, the primary outcome, by study site in Bangkok, Thailand (A) and in Harlem, New York (B), showing 4-week period and recommended regimen. Each row represents a different participant. Dark blue represents complete coverage of sex events with pre- and postsex dosing. Lighter blue shades indicate partial coverage. White represents periods where there was no coverage with pre- or postsex dosing. Black periods reflect periods where there was no sexual intercourse reported or data regarding preexposure prophylaxis use or sexual activity was missing.
In Bangkok, TFV-DP concentrations in peripheral blood mononuclear cells suggested use of ≥2 tablets on visits when sex was reported in the prior week among 97.6% in the daily arm, 98.7% in the time-driven arm (P = .60 vs daily), and 95.7% in the event-driven arm (P = .51 vs daily) (Table 2). In Harlem, TFV-DP concentrations in dried blood spots suggested use of ≥2 tablets on visits when sex was reported in the prior week among 48.5% in the daily arm, 30.9% in the time-driven arm (P = .11 vs daily), and 16.7% in the event-driven arm (P = .004 vs daily) (Table 2). Proportions taking ≥2 tablets on weeks when sex was reported did not change over time (P > .14 for all randomization groups).
Table 2.
Tablet Use and Side Effects in the HIV Prevention Trials Network (HPTN) 067 Study by Site and Arm, Bangkok, Thailand (n = 178) and Harlem, New York (n = 179)
| Characteristic | Bangkok | Harlem | ||||||
|---|---|---|---|---|---|---|---|---|
| Daily | Time-Driven | Event-Driven | P Value | Daily | Time-Driven | Event-Driven | P Value | |
| No. | 60 | 59 | 59 | … | 59 | 60 | 60 | … |
| Tablets required for full coverage | 1746 | 1573 | 1268 | .90 | 1244 | 1390 | 1582 | .55 |
| Total tablets used (% of daily) | 8285 | 3713 (44.8%) | 2157 (26.0%) | <.0001 | 5507 | 2468 (44.8%) | 2356 (42.8%) | <.0001 |
| Tablets recommended (% of daily) | 9420 | 4121 (43.7%) | 1928 (20.5%) | <.0001 | 8222 | 3674 (44.7%) | 2572 (31.3%) | <.0001 |
| Recommended tablets used (% of daily) | 8047 | 3272 (40.7%) | 1255 (15.6%) | <.0001 | 5351 | 1708 (31.9%) | 1063 (19.9%) | <.0001 |
| Adherence (tablets used / recommended) | 85.4% | 79.4% | 65.1% | <.0001 | 65.1% | 46.5% | 41.3% | <.0001 |
| Adherence category (% of group) | <.0001 | <.0001 | ||||||
| 0 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.7%) | ||
| 1%–49% | 4 (6.7%) | 2 (3.4%) | 9 (15.2%) | 20 (33.9%) | 36 (60.0%) | 40 (66.6%) | ||
| 50%–89% | 27 (45.0%) | 43 (72.9%) | 45 (76.3%) | 24 (40.7%) | 22 (36.7%) | 15 (25.0%) | ||
| 90%–99% | 25 (41.6%) | 13 (22.0%) | 2 (3.4%) | 14 (23.7%) | 0 (0%) | 0 (0%) | ||
| 100% | 4 (6.7%) | 1 (1.7%) | 2 (3.4%) | 1 (1.7%) | 0 (0%) | 1 (1.7%) | ||
| No sex | 0 (0%) | 0 (0%) | 1 (1.7%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| No interview data | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (3.3%) | 3 (5.0%) | ||
| TFV-DP concentration on weeks when sex is reporteda | ||||||||
| Median | 84.3 | 36.9 | 29.9 | <.0001 | 316.0 | 122.5 | 84.9 | .0064 |
| IQR | (64.3–127.0) | (22.9–83.0) | (18.8–46.4) | (0–942.0) | (41.8–409.0) | (0–202.0) | ||
| Visits with TFV-DP concentrations indicating ≥2 tabletsb taken per week on weeks when sex is reported. | .52 | .014 | ||||||
| Week 10 | 31/31 (100%) | 29/29 (100%) | 30/30 (100%) | 13/23 (56.5%) | 8/23 (34.8%) | 5/27 (18.5%) | ||
| Week 18 | 28/29 (96.6%) | 30/30 (100%) | 24/26 (92.3%) | 11/27 (40.7%) | 10/27 (37.0%) | 3/21 (14.3%) | ||
| Week 30 | 22/23 (95.7%) | 18/19 (94.7%) | 13/14 (92.9%) | 9/18 (50.0%) | 3/18 (16.7%) | 3/18 (16.7%) | ||
| Overall | 81/83 (97.6%) | 77/78 (98.7%) | 67/70 (95.7%) | 33/68 (48.5%) | 21/68 (30.9%) | 11/66 (16.7%) | ||
| Neurologic side effect, % of visits | 14.2% | 14.3% | 13.3% | .94 | 6.1% | 3.3% | 4.5% | .32 |
| Gastrointestinal side effects, % of visits | 13.1% | 8.5% | 10.5% | .38 | 8.0% | 5.8% | 7.1% | .75 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; TFV-DP, tenofovir diphosphate.
aTFV-DP in peripheral blood mononuclear cells (PBMCs) was analyzed for Bangkok, and dried blood spots (DBSs) for Harlem.
bFor Bangkok, TFV-DP in PBMCs >5.2 fmol/106 cells is considered as participants taken ≥2 tablets per week; For Harlem, TFV-DP in DBSs ≥326 fmol/punch is considered as participants taken ≥2 tablets per week.
Numbers of PrEP Tablets Required for Adherence and Numbers Used
Daily dosing was associated with more than twice as many tablets required for adherence to the recommended regimen (“Tablets Recommended” in Table 2) and larger numbers of tablets actually used compared to both nondaily dosing regimens. Median drug concentrations in the daily arms were nearly double the concentrations of those in the time-driven and event-driven arms when sex was reported in the prior week (P < .0001 for Bangkok, P = .0064 for Harlem; Table 2).
Adherence
In Bangkok, adherence to the recommended regimen in the daily and time-driven arms were comparable (85.4% vs 79.4%; P = .42), whereas adherence in the event-driven arm was less (65.1%; P < .0001 vs daily). In Bangkok, ≥90% adherence was evident in 29 of 60 (48.3%) participants in the daily arm vs 14 of 59 (23.7%) in the time-driven arm vs 4 of 59 (6.8%) in the event-driven arm. In Harlem, adherence in the daily arm was higher than the time-driven arm (65.1% vs 46.5%; P < .0001) and the event-driven arm (41.3%; P < .0001 vs daily). In Harlem, ≥90% adherence was evident in 15 of 59 (25.4%) in the daily arm, none of the participants in the time-driven arm, and 1 of 59 (1.7%) in the event-driven arm.
In Bangkok, sharing tablets with other trial participants who needed them was reported during 6% of interviews in the event driven arm and not in other groups. Receiving tablets from other participants because of need was reported in 3% of interviews in the daily arm and not in other groups. In Harlem, tablet sharing was reported during 1%–4% of interviews in all groups.
Self-reported Switching of Regimens
Participants were instructed to follow their specific regimen (see Supplementary Materials), although each participant received enough tablets to adopt an alternative strategy in practice. Computer-assisted self-interviews about regimen switching indicated that the following proportions of participants in Bangkok and Harlem intentionally switched regimens during the self-administered phase of the study: 12% and 10%, respectively, of the daily arm, 0 and 4%, respectively, of the time-driven arm, and 10% and 20%, respectively, of the event-driven arm after excluding missing responses (Bangkok, P = .015; Harlem, P = .037; overall P = .0010). The adopted regimen varied among people who switched.
HIV Infections
Four HIV seroconversions occurred during the study; 3 before randomization and 1 after randomization as reported previously [13]. The postrandomization seroconversion occurred after randomization to the daily arm, yet drug concentrations indicated use of <1 tablet per week at seroconversion.
Safety and Tolerability
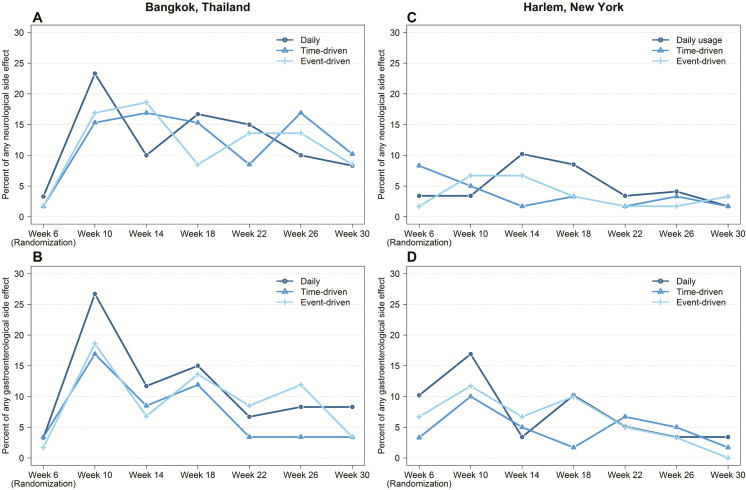
There were no significant differences in side effects by randomization group at Bangkok and Harlem related to the neurological system (P = .94 and P = .32, respectively) or gastroenterological system (P = .38 and P = .75, respectively; Table 2; Figure 4). However, there were trends toward greater side effects in the daily arm at week 10 (4 weeks after randomization) at both study sites (Figure 4). Gastrointestinal side effects became less frequent after week 10 (P < .0001 at both sites) and neurological side effects became less frequent in Bangkok (P < .017) and did not change in Harlem (P = .091). Creatinine elevations (whether confirmed or not) occurred among 16 of 178 (9.0%) participants in Bangkok (10, 2, and 4 in the daily, time-driven, and event-driven arms, respectively, P = .050) and 1 of 179 (0.5%) in Harlem in the daily arm; all were grade 1 except for 1 participant in Bangkok in the daily arm who had a grade 2 elevation. Overall, 10 of 357 (2.8%) participants temporarily or permanently discontinued PrEP due to side effects (6/178 [3.3%] in Bangkok and 4/179 [2.2%] in Harlem). Of these, 5 were in the daily arm, 4 were in the time-driven arm, and 1 was in the event-driven arm. No bone fractures were reported among participants in Bangkok and 2 fractures were reported in 2 participants in Harlem, both related to trauma.
Figure 4.
Side effects in the HIV Prevention Trials Network (HPTN) 067 study by randomization group in Bangkok, Thailand (A and B) and Harlem, New York (C and D). Neurological side effects include dizziness and headache (A and C). Gastrointestinal side effects include nausea, vomiting, and abdominal cramping (B and D).
DISCUSSION
The overall feasibility of nondaily PrEP in this study differed by study site: Nondaily dosing appeared to be feasible among men and transgender women who have sex with men in Bangkok, as was also seen in the Ipergay trial [3], whereas participants in Harlem who received a recommendation for daily dosing did substantially better in terms of coverage, adherence, and drug concentrations compared with those in Harlem who received recommendations for nondaily regimens. In contrast with MSM in Bangkok, MSM in Harlem were more similar to women in Cape Town [8], where recommendations for daily dosing also led to higher levels of coverage of sex acts.
A separate analysis of primary outcomes by site was planned based on the premise that social and cultural factors could impact behavioral outcomes, including PrEP use before and after sexual intercourse. The overall levels of retention in the study, PrEP coverage of sex events, adherence, and drug concentrations were all higher in Bangkok relative to Harlem. The participants at the Bangkok site had more years of schooling and greater employment, which may have facilitated participation in this research study. In addition, the Bangkok site provides longitudinal clinical services to large numbers of gay and bisexual men and transgender women, whereas the Harlem site is a dedicated clinical trials facility. Research settings that also provide clinical services to nonresearch clients may attract more-adherent participants or may foster greater adherence; high PrEP adherence has been observed in the context of clinical services and demonstration projects [5, 14–17]. Increased PrEP use in Bangkok may also reflect greater familiarity with PrEP among clients, more health literacy generally, more identification with gay communities, less stigma, more trust in medical services, and less access to PrEP outside the study.
Nondaily PrEP use in HPTN 067 did not decrease neurological or gastrological side effects; these symptoms are primarily reported in the first weeks of use [18, 19] and may reflect a startup syndrome rather than accumulated dose effect. Creatinine elevations occurred more frequently in Bangkok than Harlem, especially in the daily arm, likely reflecting greater PrEP use or smaller body size. The creatinine elevations were mild, nonprogressive, and did not require stopping study medication (defined per protocol as estimated creatinine clearance ≤50 mL/minute or serum creatinine ≥1.5 times the upper limit of normal). FTC/TDF PrEP decreases bone mineral density in a dose-dependent manner [20], although PrEP trials have not demonstrated an impact on bone fractures. Bone mineral density was not evaluated in this trial.
A limitation of this study is that it was conducted before the results of the Ipergay study were available. Reflecting the information available at the time, participants were informed that the efficacy of daily oral PrEP was known, whereas nondaily regimens were considered experimental, which may have undermined adherence to these regimens. Qualitative information collected during this study indicated that belief in PrEP efficacy was a powerful facilitator of adherence [21]. Providing information about the safety and efficacy of nondaily PrEP for MSM from the Ipergay study [3] may increase uptake and adherence to nondaily regimens. Another limitation is that participants were randomly assigned to the treatment arms, rather than choosing the regimen based on their frequency of sex, ability to plan for sex, and personal preference. Regimen switching was reported by a substantial minority of participants, especially from randomization to the daily and event-driven arms. This may reflect individual preferences or changes in sexual practices, which are known to change with time [22, 23]. Other limitations of the study are that the EDM device was bulky and occasionally lost (especially in Harlem), study procedures were burdensome, and visits were frequent, all of which created inconvenience that can be expected to undermine PrEP use. The duration of self-administered therapy was too short to evaluate nonpersistence of PrEP use. Too few transgender women were enrolled to allow any conclusions about their experience with daily vs nondaily regimens.
PrEP has an important role in containing the spread of HIV, as reflected in the new World Health Organization recommendation that PrEP should be offered to people at substantial risk [24]. PrEP use expanded 3-fold in the United States in 2014 [25, 26] and has been approved for support by public programs in multiple countries. Guiding people on how and when to start and stop PrEP as sexual practices and relationships change is an emerging challenge, which may be addressed with event-driven dosing for some people. For others in this study, a recommendation for daily PrEP dosing led to protective PrEP drug concentrations among the majority of men and transgender women who have sex with men in 2 markedly different settings. How well services can be adapted to diverse settings and changing sexual practices is a critical determinant of the dissemination of innovations, including PrEP, and their impact on HIV transmission.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (NIH), or the US Centers for Disease Control and Prevention.
Financial support. This work was supported by award numbers UM1 AI068619, UM1 AI068613, and RO1 AI118575 from the NIH (NIAID , National Institute of Mental Health, and National Institute on Drug Abuse). Gilead Sciences donated study medication to the NIH to support this study.
Potential conflicts of interest. R. M. G. has been a site investigator for clinical trials funded by ViiV/GSK and Gilead Sciences to the Gladstone Institutes and the San Francisco AIDS Foundation. R. A. has received an unrestricted educational grant to the University of Michigan from Gilead Sciences (2015). P. L. A. receives study drug and contract work from Gilead Sciences. C. W. H. conducts research sponsored by ViiV, GSK, and The Bill and Melinda Gates Foundation, and is a consultant at TRI and the University of California, Los Angeles. J. F. R. is employed by Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. US Public Health Service/Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014: a clinical practice guideline Available at: http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf.
- 2. European AIDS Clinical Society. EACS guidelines Available at: http://www.eacsociety.org/files/guidelines_8_0-english_web.pdf. Accessed 21 April 2016.
- 3. Molina JM, Capitant C, Spire B, et al. . On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Lerma JG, Cong ME, Mitchell J, et al. . Intermittent prophylaxis with oral Truvada protects macaques from rectal SHIV infection. Sci Transl Med 2010; 2:14ra4. [DOI] [PubMed] [Google Scholar]
- 5. Grant RM, Anderson PL, McMahan V, et al. . iPrEx Study Team Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mutua G, Sanders E, Mugo P, et al. . Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One 2012; 7:e33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kibengo FM, Ruzagira E, Katende D, et al. . Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected Ugandan volunteers living in HIV-serodiscordant relationships: a randomized, clinical trial. PLoS One 2013; 8:e74314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bekker LG, Roux S, Sebastien E, et al. . Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial [manuscript published online ahead of print 3 October 2017]. Lancet HIV 2017. doi:10.1016/S2352-3018(17)30156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. R Amico K, McMahan V, Goicochea P, et al. . Supporting study product use and accuracy in self-report in the iPrEx study: next step counseling and neutral assessment. AIDS Behav 2012; 16:1243–59. [DOI] [PubMed] [Google Scholar]
- 10. Castillo-Mancilla JR, Zheng JH, Rower JE, et al. . Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson PL, Glidden DV, Liu A, et al. . Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73:13–22. [Google Scholar]
- 13. Sivay MV, Li M, Piwowar-Manning E, et al. . HPTN 067/ADAPT Study Team Characterization of HIV seroconverters in a TDF/FTC PrEP study: HPTN 067/ADAPT. J Acquir Immune Defic Syndr 2017; 75:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henderson F, Taylor A, Chirwa L, et al. . Characteristics and oral PrEP adherence in the TDF2 open-label extension in Botswana. In: Eighth International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention, Vancouver, Canada, 19–22 July 2015. [Google Scholar]
- 15. McCormack S, Dunn DT, Desai M, et al. . Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu AY, Cohen SE, Vittinghoff E, et al. . Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016; 176:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin M, Vanichseni S, Suntharasamai P, et al. . Bangkok Tenofovir Study Group Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir Study. Lancet HIV 2017; 4:e59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grant RM, Lama JR, Anderson PL, et al. . iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baeten JM, Donnell D, Ndase P, et al. . Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mulligan K, Glidden DV, Anderson PL, et al. . Preexposure Prophylaxis Initiative Study Team Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2015; 61:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chemnasiri T, Varangrat A, Amico K, et al. . Facilitators and barriers affecting PrEP adherence among Thai men who have sex with men in the HPTN 067/ADAPT study, a qualitative analysis. In: Eighth International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention, Vancouver, Canada, 19–22 July 2015. [Google Scholar]
- 22. Carlo Hojilla J, Koester KA, Cohen SE, et al. . Sexual behavior, risk compensation, and HIV prevention strategies among participants in the San Francisco PrEP demonstration project: a qualitative analysis of counseling notes. AIDS Behav 2016; 20:1461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grant RM, Glidden DV. HIV moments and pre-exposure prophylaxis. Lancet 2016; 387:1507–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 25. Bush S, Ng L, Magnuson D, Piontkowsky D, Mera Giler R. Significant uptake of Truvada for pre-exposure prophylaxis (PrEP) utilization in the USA in late 2014—1Q 2015. In: International Association of Providers of AIDS Care Treatment, Prevention, and Adherence Conference, Miami, Florida, 2015. [Google Scholar]
- 26. Grant R, Hecht J, Raymond H, et al. . Scale-up of pre-exposure prophylaxis in San Francisco to impact HIV incidence [abstract 25]. In: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.