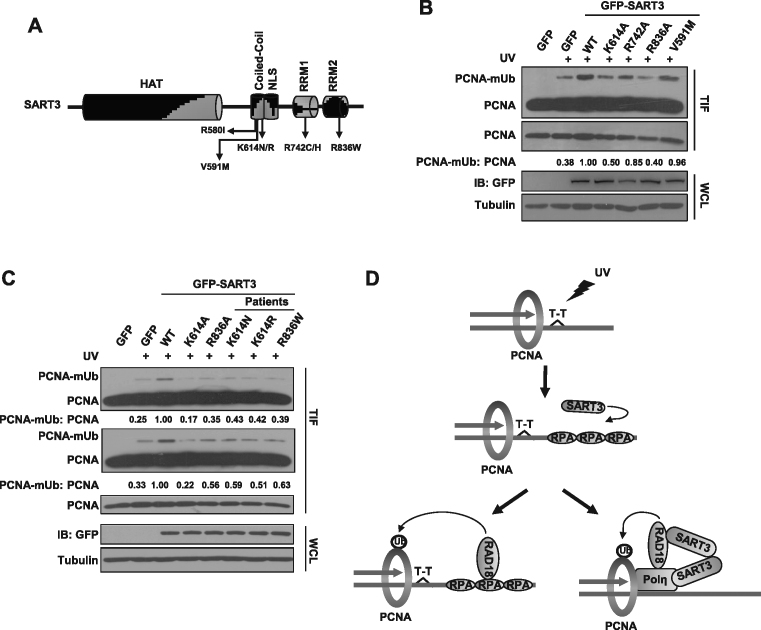
Figure 7.
Cancer-associated SART3 mutants affect TLS. (A) Schematic representation of SART3 mutants with repeated incidence found in tumor samples. (B) Two SART3 mutants exhibit lower efficiency in promoting PCNA-mUb. U2OS cells were transfected with a series of GFP-SART3 mutants (targeted amino acids were mutated into alanine), then the cells were treated with 15 J/m2 UVC and incubated for 4 h. The triton-insoluble fractions (TIF) and whole-cell lysates (WCL) were harvested and checked with antibodies against PCNA, GFP and Tubulin. Tubulin: loading control. Levels of PCNA and PCNA-mUb were quantified using ImageJ. (C) Several SART3 mutants with repeated incidence from cancer patients manifest impaired PCNA-mUb formation as compared to that of WT. U2OS cells were transfected with a series of GFP-SART3 mutants (found in patients), then treated with 15 J/m2 UVC and further cultured. The triton-insoluble fractions (TIF) and whole-cell lysates (WCL) were harvested and separated by SDS-PAGE. Levels of PCNA and PCNA-mUb were quantified using Image J. (D) The working model of how SART3 regulates TLS after UV irradiation. SART3 functions in TLS at dual mode. On the one hand, SART3 facilitates ssDNA generation followed by promoting RPA and RAD18 recruitment. On the other hand, SART3 enhances RAD18/Polη interaction via its homodimerization, which synergistically promotes PCNA-mUb formation, modulating TLS.