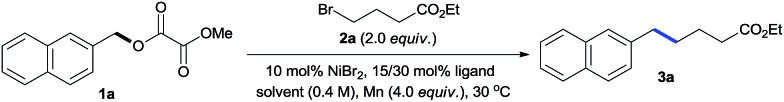
Table 1. Nickel-catalyzed reductive coupling of 1a with 2a a .
| |||
| Entry | Ligand | Solvent | Yield (%) |
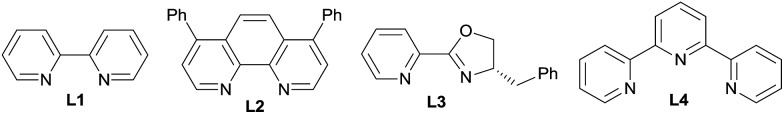
| 1 | L1 | DMF | 20 |
| 2 | L2 | DMF | 18 |
| 3 | L3 | DMF | 32 |
| 4 | L4 | DMF | 0 |
| 5 | PPh3 | DMF | 34 |
| 6 | P(4-MePh)3 | DMF | 16 |
| 7 | P(2-MePh)3 | DMF | 0 |
| 8 | P(4-FPh)3 | DMF | 50 |
| 9 | P(4-CF3Ph)3 | DMF | 58 |
| 10 | P(4-CF3Ph)3 | DMA | 52 |
| 11 | P(4-CF3Ph)3 | DMSO | 73 (79) b |
| 12 | P(4-CF3Ph)3 | DMSO/DMA 1 : 1 | 68 (73) b |
| 13 | P(4-CF3Ph)3 | DMSO/DMF 1 : 1 | 70 (75) b , (82) c |
| 14 d | P(4-CF3Ph)3 | DMSO/DMF 1 : 1 | 0 |
| 15 e | P(4-CF3Ph)3 | DMSO/DMF 1 : 1 | 0 |
| |||
aSubstrates 1a (0.2 mmol), monodentate ligand (30 mol%), or bidentate ligand (15 mol%) were used and reacted for 24 h; yields were determined by 1H NMR using anisole as an internal standard.
bYields are isolated yields.
c 1a (4 mmol, 0.976 g) was used; isolated yield.
dNo Ni catalyst.
eNo Mn.
