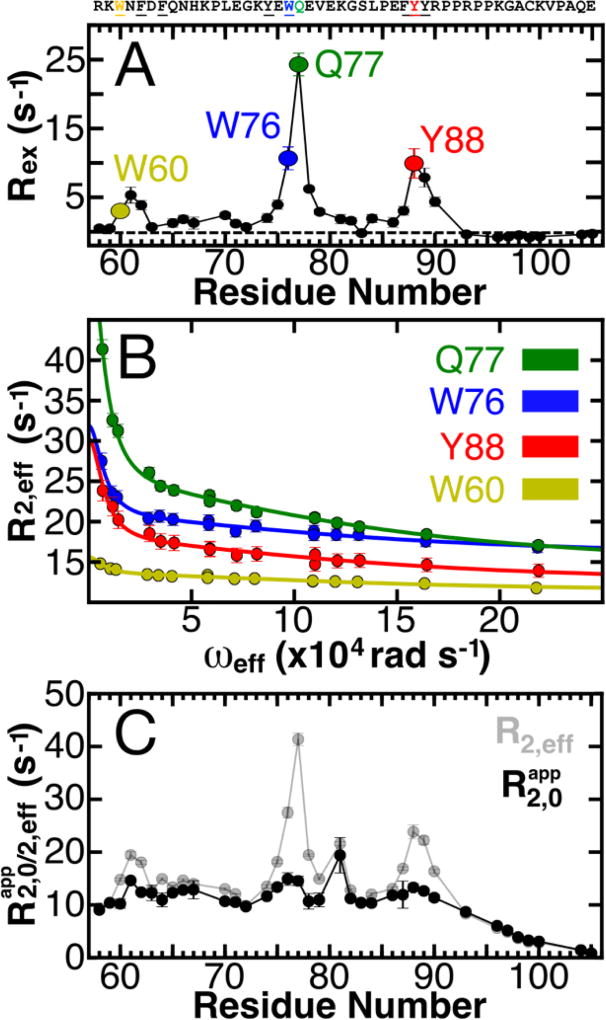
Figure 1. Defining the conformational landscape of p27-D2.
(A) Large effective refocusing field strength (ωeff) and low temperature 1HN relaxation dispersion (RD) experiments reveal multi-state conformational exchange for the intrinsically disordered protein, p27-D2. Conformational exchange is sensed by many residues within p27-D2, which exhibit quantifiable exchange contributions ( ; the dashed line indicates a Rex value of zero). The greatest observed conformational exchange is observed for residues in p27-D2 within three aromatic clusters, including W60 (yellow), W76 (blue), and Y88 (red); the 1HN displaying the largest amplitude of motion is Q77 (green). (B) The observed RD monitors the change in the effective transverse relaxation rate (R2,eff) as a function of ωeff and reports that some residues undergo conformational exchange that has two kinetic phases. Solid lines correspond to global fits of all RD data to a thermodynamic model that describes nuclei which sense two separate kinetic phases. Data presented in A & B were collected at 274 K. (C) Plot of R2,eff measured at low ωeff (grey points) where a maximal contribution of microsecond exchange is detected as compared to the transverse relaxation rate due to the apparent intrinsic fast nanosecond exchange ( ; black points) across all residues. was determined from the fitted RD data and shows that large amplitude 1HN RD permits extensive sampling of Rex by using high ωeff which substantially quenches RD. The fast microsecond exchange detected in p27-D2 accounts for the elevated R2,eff values and once these are taken into account, a flat, featureless profile is observed as expected for a prototypical disordered protein.