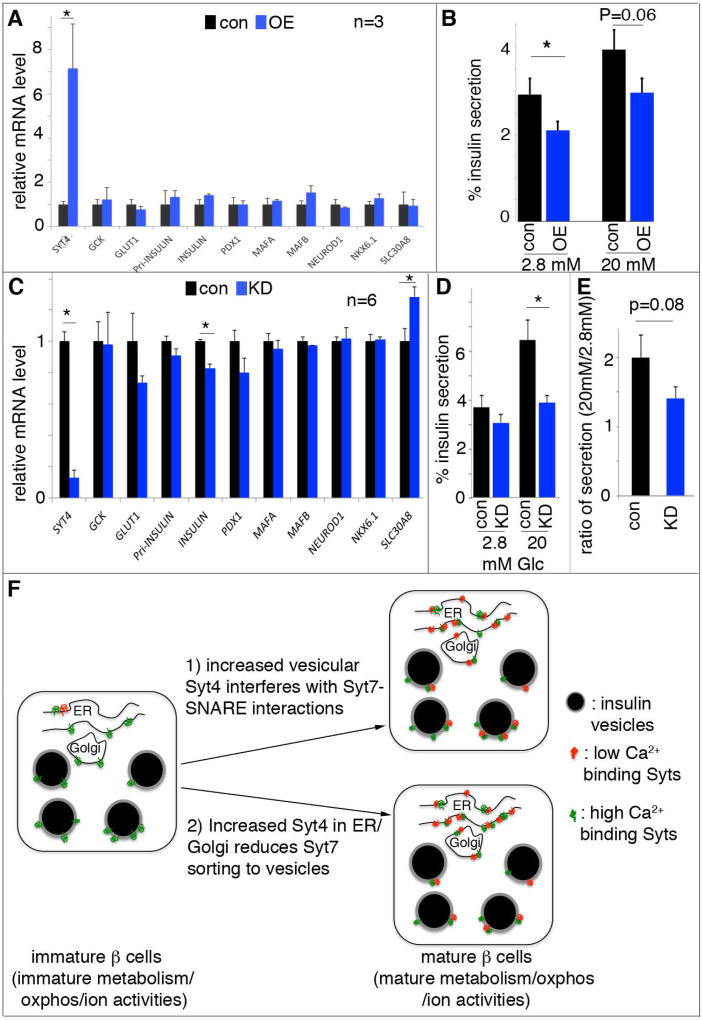
Figure 7. SYT4 regulates human β-cell GSIS.
Lentivirus was used to introduce human SYT4 overexpression, while siRNA were used for SYT4 knockdown. (A) Gene expression in EndoC-βH1 cells with SYT4OE (OE), normalized against empty virus-infected control (con) cells. (B) GSIS in EndoC-βH1 cells with SYT4OE. (C) Gene expression in EndoC-βH1 cells with SYT4KD (KD), normalized against cells treated with scrabbled siRNA (con). (D, E) Insulin secretion in EndoC-βH1 cells with SYT4KD, presented as the % of insulin secretion at different level of glucose (D), or as the ratio of insulin release at 20 mM over 2.8 mM glucose (E). *: P<0.05. (F) A model with two pathways that converge to regulate β-cell maturation: one aspect includes glucose metabolism/subsequent oxidative phosphorylation and membrane excitability, which ensure efficient glucose metabolism, ATP production, ionic activity, and Ca2+ entry. Another is the modulation of Ca2+ sensitivity of the vesicles. In this pathway, immature β cells have lower levels and mature β cells have higher levels of non-Ca2+ binding Syts. The Ca2+-binding Syts remain unchanged during maturation. The increased ratio between non-Ca2+ to Ca2+ binding Syts can desensitize the vesicles so that high secretion only occurs at high Ca2+ in the mature β cells. This desensitization can be achieved by 1) using Syt4 localized on vesicles to inhibit Syt7-SNARE interactions, 2) reducing Syt7 levels on vesicle surface, or both.