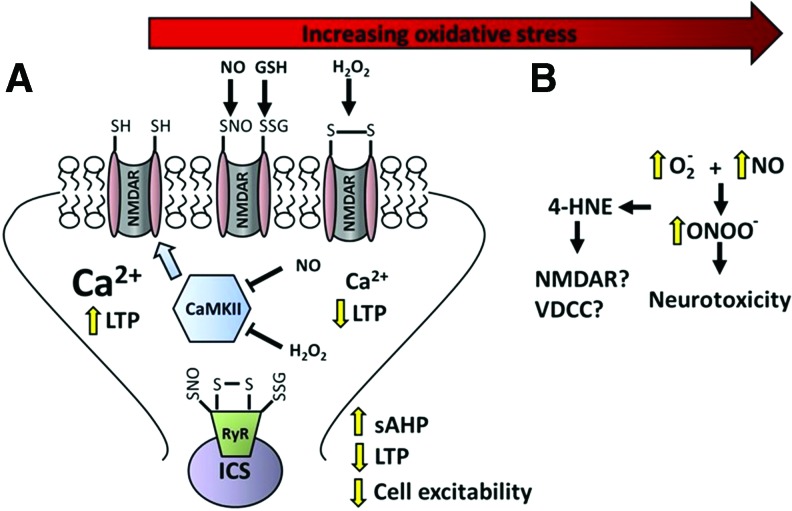
FIG. 8.
Molecular mechanisms for progression from normal to senescent physiology and neurotoxicity with increasing oxidative stress. (A) Binding of glutamate and postsynaptic depolarization results in NMDAR activation and an influx of Ca2+ to induce LTP. The increase in intracellular Ca2+ initiates a modest and reversible increase in ROS, nitric oxoide (NO), and/or hydrogen peroxide (H2O2). In turn, ROS interacts with redox-sensitive cysteines to induce reversible formation of disulfide bonds, S-glutathionylation, or S-nitrosylation (SNO), inhibiting NMDAR activity. The temporary reduction in NMDAR activity may permit the stabilization of synaptic plasticity during learning. If ROS levels are prolonged, NMDARs remain hyporesponsive due to redox changes at the NMDAR and reduced activity of CaMKII. Similar redox modifications of RyRs increase release of Ca2+ from ICS, increasing the amplitude of sAHP. In turn, the sustained hyperpolarizing response further inhibits voltage-dependent NMDAR activation, contributing to impaired LTP induction. (B) As oxidative stress increases, lipid peroxidation and the formation of 4-HNE may have effects on NMDARs and VDCCs that result in a further dysregulation of Ca2+ homeostasis. In addition, increased levels of superoxide and the formation of peroxynitrite produce oxidative damage of vulnerable molecules, resulting in an irreversible inhibitory action on proteins and neurotoxicity. ICS, intracellular Ca2+ stores; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars