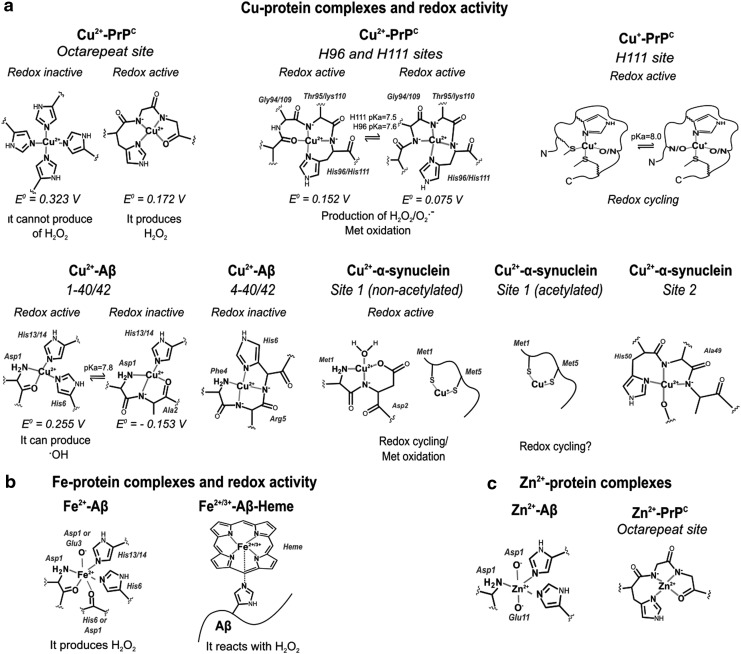
FIG. 3.
Structural features and redox properties of relevant metal-protein complexes in neurodegenerative diseases. (a) Cu can interact with PrPC, Aβ, and α-synuclein. PrPC has three coordination sites: histidine (His) His96, His111, and an octarepeat. Cu2+ coordination to His96 and His111 yields very similar complexes different to those generated upon Cu+ binding to His111. The octarepeat region coordinates up to four Cu2+ ions depending on the Cu2+/PrPC stoichiometric ratio. At low Cu:protein ratios, four His residues coordinate Cu2+, while at high ratios each His can bind one Cu2+ ion. The different coordination chemistry of these Cu-PrPC complexes is reflected in their redox properties. Similarly, Cu2+ coordination to Aβ (1–40/42) yields two different coordination modes that display different redox properties. Recently, coordination to Aβ (4–40/42) has also been described; this site displays higher affinity for Cu2+ than Aβ (1–40/42), and it yields a redox silent complex. On the contrary, nonacetylated α-synuclein has two high-affinity binding sites for Cu2+: site one involving residues at the N-terminal, and site two at His50. Acetylation of α-synuclein, as described to occur in vivo, abolishes Cu2+ binding at site 1, although both forms of α-synuclein can bind Cu+. (b) Fe2+ can interact with Aβ, but the redox properties of Fe-Aβ complexes remain unclear. Interestingly, recent studies propose that heme can bind to Aβ, yielding a heme (Fe)-Aβ complex with peroxidase activity. (c) Finally, Zn2+ can bind to the octarepeat region of PrPC and with Aβ. Cu2+, cupric ion; Fe, iron; Fe2+, ferrous; Zn, zinc.