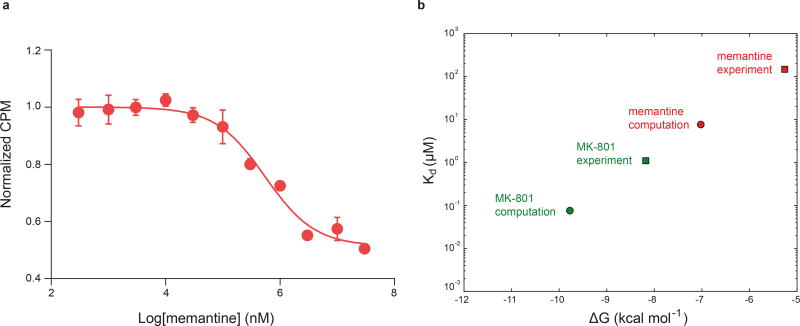
Extended Data Figure 7. Free energy estimates of MK-801 and memantine binding.
a, Competition binding of memantine to the ∆2 receptor in the presence of 3 μM 3H MK-801, measured by the scintillation proximity assay. The plot shows data from a representative experiment with error bars representing s.e.m. from triplicate measurements. b, Dissociation constants (Kd, circles), derived from free energy estimates of binding of memantine and MK-801 to the open, intact, activated ∆2 receptor in which the pore has collapsed onto the ligand. The absolute experimental affinities of MK-801 (green) and memantine (red) for the ∆2 receptors are shown as squares. The free energies were calculated for four independent, ligand-bound configurations, all taken from binding simulations at zero transmembrane voltage (MK-801: Sims. 20 and 21; memantine: Sims. 24, 25, and 27). Each calculation consisted of 0.5 µs of simulation to solvate the ligand in water, followed by 3.0 µs of simulation of the protein-ligand complex. The average Kd values for MK-801 and memantine of ≈ 0.08 and ≈ 7.64 µM for the ∆2 receptor show a 100-fold difference in the affinities of these two ligands; the similar relative affinity of these two ligands has been found experimentally against the ∆2 receptor with Kd value of ≈ 1.1 μM for MK-801 and Ki value of ≈ 147.4 μM for memantine. The calculated binding free energies −9.78 ± 1.61 (MK-801) and −7.02 ± 1.24 kcal mol−1 (memantine), and thus the free energy–derived dissociation constants, are subject to large errors, estimated as standard errors of the mean, due to lack of convergence of including long-range effects from lipid molecules surrounding the pore. We note that the contribution of pore-cavity collapse upon ligand binding to the binding free energy is not included in the free energy calculations, which were performed with the pore-collapsed, intact, agonist-bound receptor. Also not included is the contribution of −ln(2)kBT arising from the two poses available to MK-801.