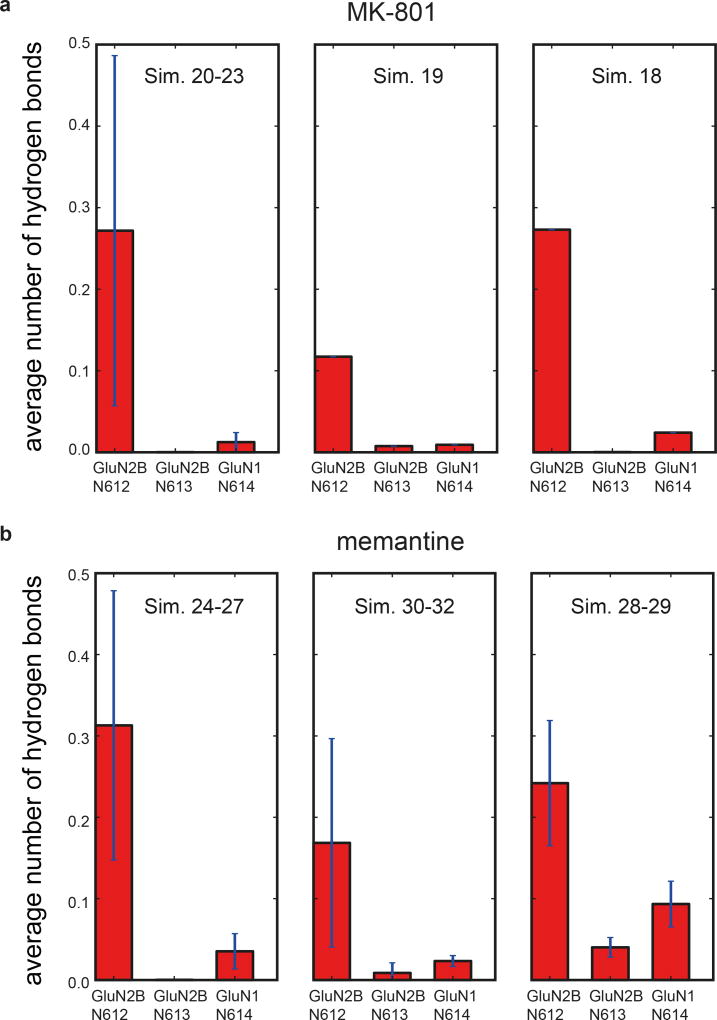
Extended Data Figure 8. Hydrogen bonding propensity between MK-801 (a) and memantine (b) and the selectivity filter asparagine residues.
a-b, The two N-site asparagine residues N614 (GluN1) and N612 (GluN2B) of the pore-loop tips, and the N+1 asparagine residue, N613, of the GluN2B subunit, which is believed to be involved in the voltage dependence of memantine binding. For MK-801 (a), the first panel shows data obtained at zero transmembrane voltage (Sims. 20–23), the second at 197.9 ± 1.2 mV (Sim. 19), and the third at 593.9 ± 3.8 mV (Sim. 18). N=4, N=1, N=1; n>>10. For memantine (b), the first panel shows data obtained at zero transmembrane voltage (Sims. 24–27), the second at 196.9 ± 0.1 mV (Sims. 30–32), and the third at 592.6 ± 0.3 and 592.7 ± 0.3 mV (Sims. 28 and 29). N=4, N=3, N=2; n>>10. Hydrogen bonding propensity is relatively low for N614 of GluN1, except at high voltage, suggesting that N612 and N613 of GluN2B are more important for pore-blocker binding and its voltage dependence, respectively; at nonzero transmembrane voltage, hydrogen bonding propensity increases at the N+1 site asparagine N613 of GluN2B. The error bars are standard deviations of the mean calculated from all individual data points aggregated across all simulations.