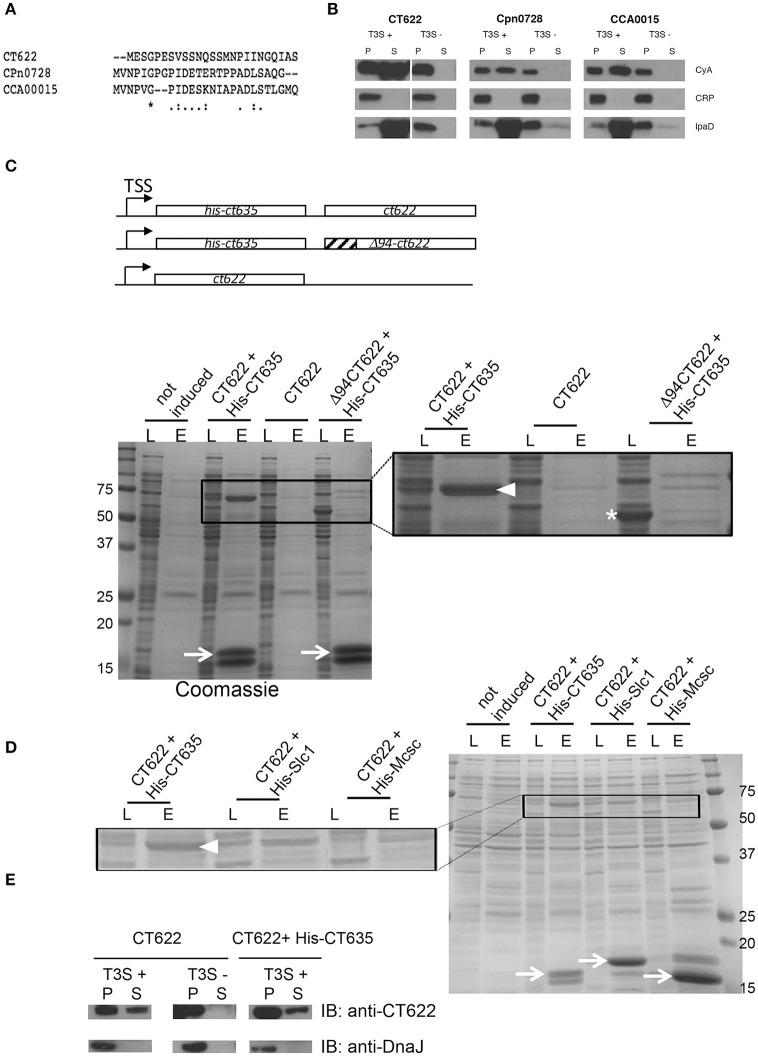
Figure 1.
CT622 and its homologs are T3S substrates. (A) The first 25 amino acids of CT622 and its homologs in C. pneumoniae (CPn0728) and C. caviae (CCA00015) are shown. (B) The amino-terminal segments of the indicated proteins were fused to the Cya reporter protein and expressed in a S. flexneri ipaB (constitutive T3S) or mxiD (defective T3S) strain. Exponential phase cultures expressing the reporter fusion protein were fractionated into supernatants (S) and pellets (P). Samples were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with anti-Cya (to detect chlamydial fusion proteins), anti-IpaD (Shigella secreted protein), or anti-CRP (Shigella non-secreted protein) antibodies. (C) Top: schematic view of the plasmids used. Bottom: CT622 co-purify with CT635. E. coli were transformed with a plasmid expressing CT622 and HIS-CT635 (lanes 1–4), CT622 alone (lanes 5, 6), or CT622 truncated of 94 amino acids and HIS-CT635 (lanes 7,8). HIS-tagged proteins were pulled-down from bacterial lysates using nickel-coupled beads. Whole bacterial lysates (L) and eluted fractions (E) are shown. Samples were resolved by SDS-PAGE and strained with Coomassie blue, arrows point to the HIS-tagged chaperone (expected mw = 18 kDa). The inset shows a two-fold enlargement, with arrowhead pointing to CT622 in the elution fraction when co-expressed with HIS-CT635, and the asterisk to Δ94CT622. (D) Same as in C, with E. coli transformed with a plasmid expressing CT622 and His-CT635 (lanes 1–4), CT622 and His-Slc1 (lanes 5, 6), or CT622 and His-Mcsc (lanes 7,8). Arrows point to the His-tagged proteins, arrowhead to CT622. The inset shows a two-fold enlargement. (E) The indicated S. flexneri strains were transformed with a plasmid expressing CT622 alone or together with HIS-CT635. Translocation of CT622 in the culture supernatant (S) was analyzed as described in (B), with the exception that antibodies against DnaJ were used to control for bacterial lysis.