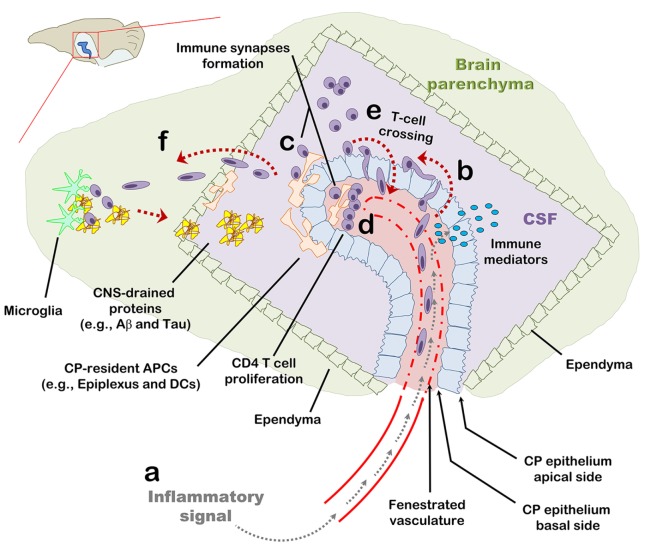
Figure 6.
The choroid plexus (CP) as a checkpoint for cell-mediated immunity in the central nervous system (CNS): a suggested model. The CP manufactures most of the cerebrospinal fluid (CSF) and serves as an interface between the blood and the CNS. (A) The CP primarily comprises a fenestrated vasculature, a stroma, and epithelial, whose apical surfaces face the CSF. Inflammatory signals such as IL-1β and tumor necrosis factor activate the CP vasculature and epithelium and induce immune signaling in the CP compartment. (B) As part of this inflammatory reaction, peripheral blood effector and/or memory T cells are recruited to the CP stroma and into the CSF. (C,D) Antigens in the CNS, either self or foreign, which drain into the CSF, are sampled by antigen-presenting cells and presented to CD4 T cells which, thereby, undergo activation and migrate into the CNS parenchyma. (E) Intercellular adhesion molecule 1 and chemokines strongly upregulated at the apical surface of the CP epithelium allow T cells in the CSF to adhere the CP and cross its epithelium back into the CP stroma. (F) Activated CD4 T cells further facilitate cell-mediated immunity in the CNS by preconditioning the CNS for cell migration across the ependymal layer of the ventricle and/or across the parenchymal and meningeal CNS vasculature. T-cell activation in the CP compartment, may not only serve as a checkpoint for cell-mediated immunity in the CNS but also impact the immune network required for brain functioning and repair at steady-state.