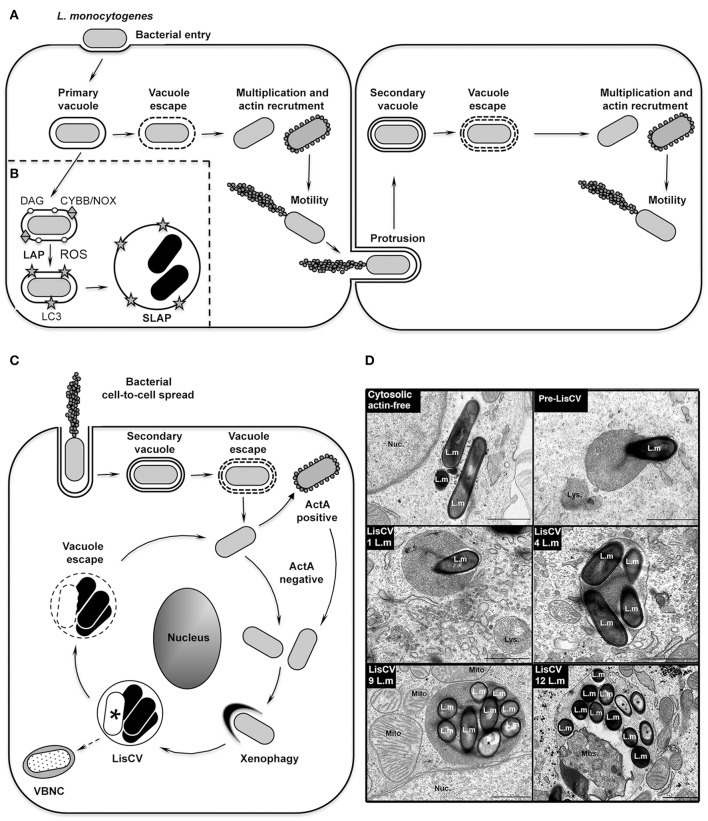
Figure 1.
The intracellular life cycle of L. monocytogenes. (A) Intracellular invasion and dissemination of L. monocytogenes in mammalian cells (adapted from Portnoy, 2012). After bacterial entry into the host cell and transient residence within a primary vacuole, bacteria escape into the cytoplasm, multiply and induce expression of the actin-polymerizing factor ActA. Actin polymerization promotes bacterial motility and cell-to-cell spread via the generation of membrane protrusions from the primary infected cell to neighboring cells. After the resolution of these protrusions into double-membrane secondary vacuoles, from which the bacteria escape, a new cycle of infection is initiated. (B) Model of SLAP formation in murine macrophages (adapted from Lam et al., 2013). After bacterial phagocytosis, subpopulations of bacteria expressing low amounts of the cytolysin LLO remain in phagosomes. Diacylglycerol accumulates on these phagosomes, as a result of bacterial (PLC) and host (PLD and PPAP2A) enzymatic activities. DAG accumulation stimulates activation of the CYBB/NOX2 NADPH oxidase and the production of reactive oxygen species (ROS), which subsequently induce recruitment of LC3 to the phagosome. This LC3-associated-phagocytosis (LAP) process is not harmful for Listeria and promotes formation of SLAPs, in which bacteria enter a slow/non-replicative state (black bacteria). (C) Model of LisCV formation in human hepatocytes and trophoblast cells (adapted from Kortebi et al., 2017). Following the active stage of bacterial cell-to-cell spread (as in A), bacteria do not re-express ActA after escape from the secondary vacuole, or stop expressing ActA after a transient ActA-positive cytosolic stage. ActA-free bacteria multiply in the cytosol and are captured by a xenophagy-like process, forming Listeria-containing vacuoles (LisCV). In these lysosome-like compartments, subpopulations of bacteria resist stress and degradation and enter a slow/non-replicative state (black bacteria), while others are sensitive to stress and die (white bacteria with a “*”). Upon unidentified stimuli, bacteria may egress from vacuoles and re-initiate a novel cycle of actin-polymerization following re-expression of ActA. In absence of a reactivation signal, wild type bacteria may behave like ΔactA bacteria, which enter the VBNC state (punctuated bacteria). (D) TEM micrographs illustrating different steps of LisCV formation in JEG3 cells infected for 72 h. Shown are the actin-free cytosolic stage, the capture of a bacterium by uncharacterized membranous electron-dense compartment (“pre-LisCV”) and the vacuolar stage with LisCVs containing different numbers of bacteria. Damaged bacteria are indicated with a “*”. L.m, L. monocytogenes; Nuc., nucleus; Lys., secondary lysosomes; Mito., mitochondria; Mbs., membranous intravacuolar structures (Adapted from Kortebi et al., 2017).