Table 1.
Structure-activity relationships for substituted 4-anilino-2-(2-methoxyanilino)-7H-pyrrolo[2,3-d]pyrimidine inhibition of TSSK2 activity
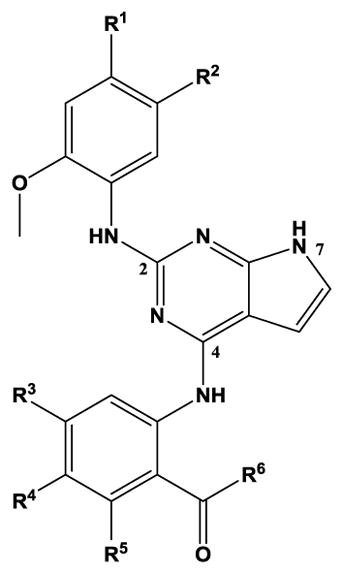
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | TSSK2 IC50, nM[a] |
| 1 |
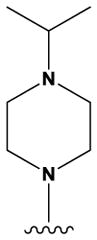
|
H | H | F | H | NH2 | 72 ± 10 |
| 2 |

|
H | H | F | F | NH2 | 150 ± 30 |
| 3 |

|
H | H | F | H |
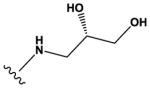
|
1200 ± 200 |
| 4 |

|
H | H | H | F | OH | 2000 ± 200 |
| 5 |
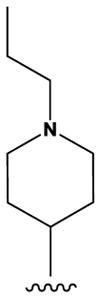
|
H | F | H | H | NH2 | 107 [b] |
| 6 |
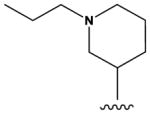
|
H | F | H | H | NH2 | 250 ± 50 |
| 7 | CH3 |
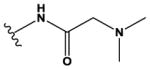
|
H | H | F | NH2 | 280 ± 60 |
| 8 | CH3 |
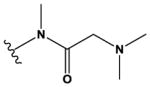
|
H | H | F | NH2 | 14,000 ± 2000 |
| 9 | Cl |
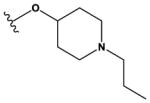
|
H | H | F | NH2 | 1300 ± 200 |
Mean ± SEM of at least 3 independent experiments.
N = 2 due to limited sample availability.
