Table 3.
Structure-activity relationships for substituted N2,N4-diphenyl-2,4-pyrimidinediamine inhibition of TSSK2
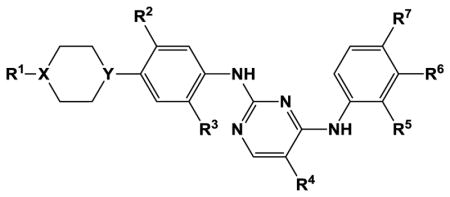
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R1 | X | Y | R2 | R3 | R4 | R5 | R6 | R7 | TSSK2 IC50, nM[a] |
| 17 | Me | N | N | H | OMe | Br |
|
H | H | 31 ± 7 |
| 18 | Me | N | N | H | OMe | Cl |

|
H | H | 37 ± 5 |
| 19 | Me | N | CH | H | OMe | Cl |

|
H | H | 66 ± 8 |
| 20 | - | O | N | H | OMe | Cl |
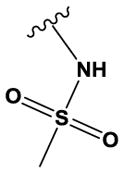
|
H | H | 750 ± 110 |
| 21 | - | O | N | H | OMe | F |

|
H | H | 13,000 ± 2000 |
| 22 |
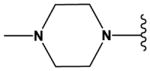
|
CH | N | H | OMe | Cl |
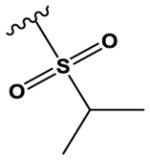
|
H | H | 80 ± 13 |
| 23 | H | N | CH | Me | OiPr | Cl |

|
H | H | 610 ± 30 |
| 24 | N(CH3)2 | CH | N | H | OMe | Cl |
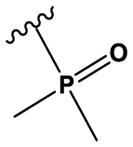
|
H | H | 230 ± 30 |
| 25 |

|
CH | N | H | OMe | Cl |

|
H | H | 510 ± 80 |
| 26 | H | N | N | H | H | Cl |
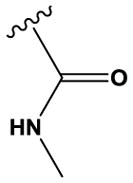
|
H | H | 963 ± 7 |
| 27 | - | O | N | H | OMe | Cl |

|
H | H | 7600 ± 800 |
| 28 | - | O | N | H | H | Cl |

|
H | H | > 100,000 |
| 29 | H | CH | N | H | H | H | COOH | H | H | > 100,000 |
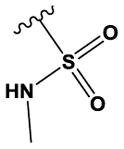
| 30 | Me | N | N | H | H | Me | H |
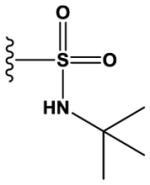
|
H | 6200 ± 900 |
| 31 |
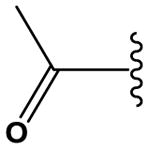
|
N | N | H | OMe | CF3 | H |
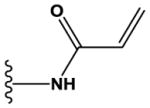
|
H | 15,000 ± 1000 |
| 32 |

|
N | N | H | OMe | Cl | OMe | H |
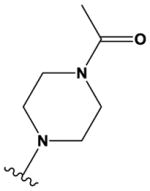
|
62,000 ± 4000 |
| 33 | - | O | N | H | H | F | H | H |
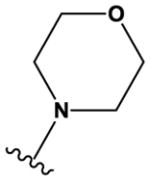
|
> 100,000 |
Mean ± SEM of at least 3 independent experiments.
