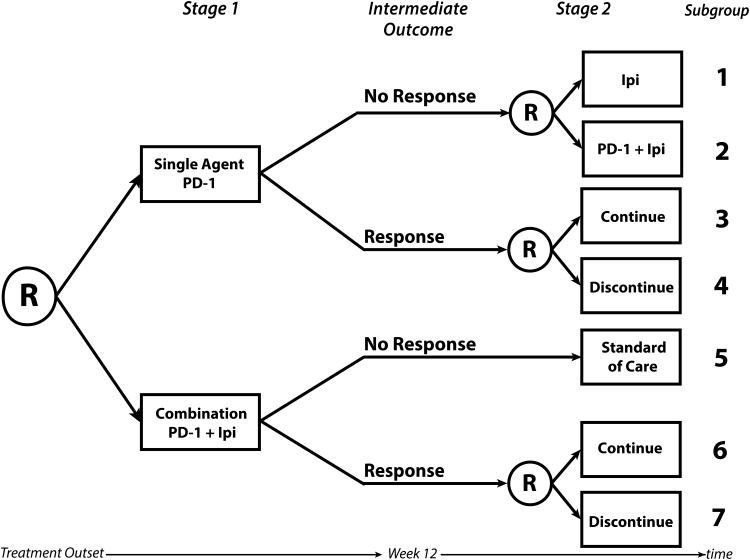
Figure 2.
A hypothetical two-stage sequential multiple assignment randomized trial (SMART) design in the setting of BRAF wild-type metastatic melanoma. Participants are initially randomized to either single agent anti-programmed cell death protein 1 (PD-1) therapy or to a combination of anti-PD-1 therapy plus ipilimumab (Ipi). Note that ipilimumab may be replaced by any novel combination agent. After four doses or approximately 12 weeks, response is measured. Those who did not respond to the single agent are re-randomized to receive ipilimumab or the combination. Those who did respond to single agent anti-PD-1 are re-randomized to continue the single agent or discontinue therapy. Those who did not respond initially to the combination receive standard of care and those who did respond are re-randomized to continue the combination or discontinue therapy. Subgroups 1-7 denote the subgroups that any one participant may fall into. There are 6 embedded treatment pathways in this SMART, each one made up of 2 subgroups: {1,3}, {1,4}, {2,3}, {2,4}, {5,6}, and {5,7}.