Fig 3. Resonance assignment of the Httex1 monomer suggests presence of transient structure.
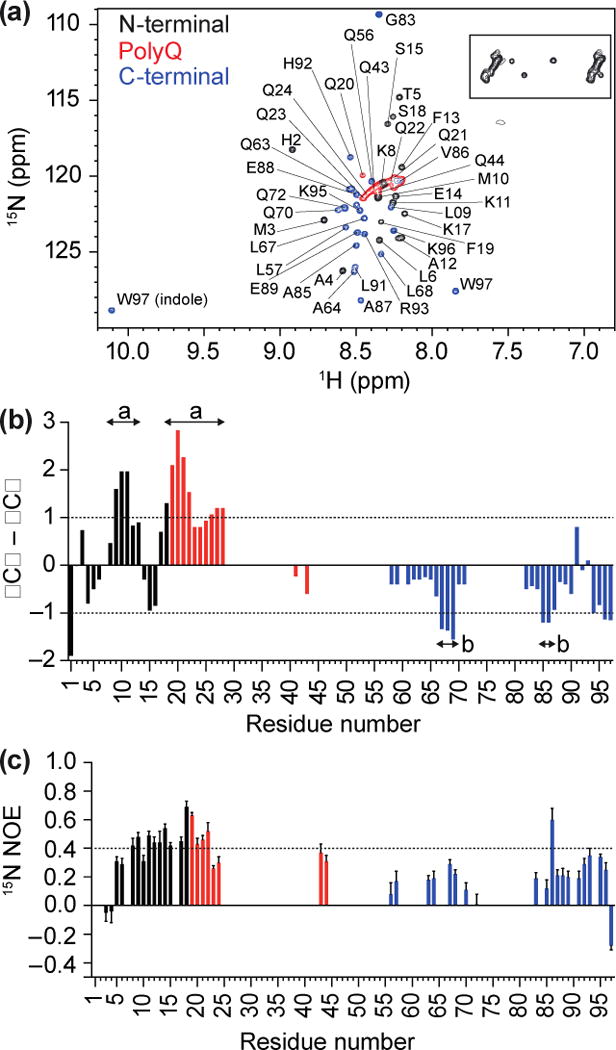
(a) 1H,15N HSQC of Httex1 protein fragment (25Q) in sodium acetate buffer (150 mM; pH 4), recorded at 5 °C, indicating N-terminal, polyQ and C-terminal Httex1 backbone amide peaks, with glutamine side chains (boxed). (b) Analysis of propensity of secondary structure based on ΔCα–ΔCβ values where persistence of positive ΔCα–ΔCβ differences suggest α-helical structures and persistence of negative ΔCα–ΔCβ differences are consistent with β-strands. (c) 15N{1H}-NOE revealed positive NOE values of 0.4 to 0.6 for N-terminal Httex1 25Q and part of the polyQ region.
