Table 1.
Exploration of VCP substrate scope
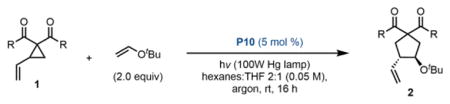
| ||||||
|---|---|---|---|---|---|---|
| entrya | R = | solventb | yield (%)c | dr [trans:cis)d | er (trans)e | er (cis)e |
| 1f,g |
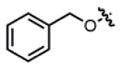 2-ester |
THF | 44 | 78:22 | 47:53 | ND |
| 2 |
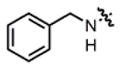 2a |
THF | 70 | 40:60 | 83:17 | 72:28 |
| 3 |
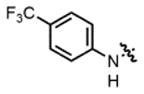 2b |
hex/THF | 84 | 79:21 | 90:10 | 75:25 |
| 4 | THF | 82 | 77:23 | 88:12 | 76:24 | |
| 5f | MeOH | 82 | 76:22 | 70:30 | 65:35 | |
| 6 |
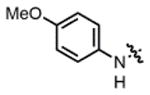 2c |
THF | 79 | 68:32 | 85:15 | 75:25 |
| 7 |
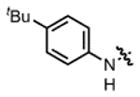 2d |
hex/THF | 32 | 68:32 | 86:14 | 75:25 |
| 8 |
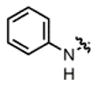 2e |
hex/THF | 77 | 73:27 | 90:10 | 79:21 |
| 9 |
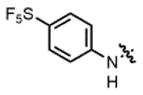 2f |
hex/THF | 83 | 80:20 | 90:10 | 76:24 |
| 10 |
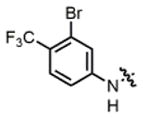 2g |
hex/THF | 81 | 82:18 | 90:10 | 73:27 |
| 11 |
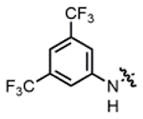 2h |
hex/THF | 94 | 82:18 | 90:10 | 73:27 |
Reactions were performed on a 0.1 mmol scale and were quenched by exposure to air. Reported data is the average of 2 separate experiments, unless noted.
Standard conditions: 2:1 hexanes:THF.
Isolated yield of a mixture of trans and cis diastereomers.
Determined by 1H NMR.
Determined by CSP-HPLC (254 nm, uncorrected).
Single experiment.
dr is crude before purification to remove cis, yield is pure trans.
