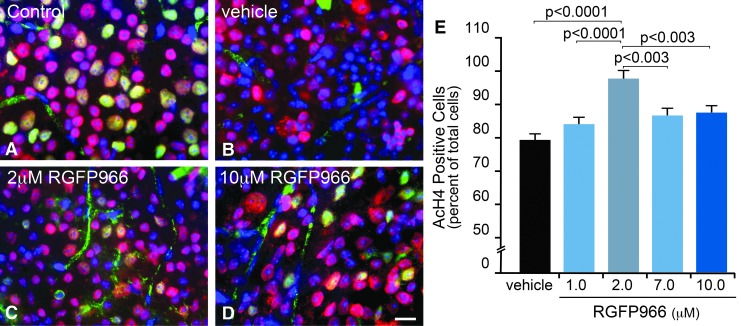
FIG. 1.
Intravitreal injection of RGFP966 prevents histone H4 deacetylation when administered at a 2.0 μM concentration to the vitreous. Mice were injected intravitreally with 30% HPβCD, 0.1 M acetate (pH 5.4) vehicle, 1.0, 2.0, 7.0, or 10.0 μM RGFP966 immediately following ONC surgery. Five days later, retinas were whole mounted and fluorescently labeled for AcH4 (Texas Red), BRN3A (Alexa-488), and DNA with DAPI. (A) Uninjured untreated control OD retinas contained healthy, BRN3A (green), and AcH4 (red) positively labeled cells in the GCL. Note that blood vessels in the GCL are nonspecifically labeled by the BRN3A antibody. (B) OS retinas that were treated with vehicle showed a reduction in AcH4 positively labeled cells and exhibited a visible loss of expression of BRN3A, which is consistent with previous evidence that RGC-specific gene silencing occurs shortly after ONC.2 (C, D) OS retinas that were treated with 2.0 or 10.0 μM RGFP966 retained more AcH4 and BRN3A-positive cells than vehicle-injected retinas following ONC. Scale bar = 10 μm. (E) Cell counts for the dose–response curve indicated that significant abolition of the histone deacetylation response resulted from single treatment with an HDAC3-specific inhibitor immediately following ONC, with the highest percentage of AcH4-positive cells present in the 2.0 μM treatment paradigm (P < 0.0001, relative to vehicle and 1.0 μM; P < 0.003, relative to 7.0 and 10.0 μM). AcH4, acetylated histone 4; BRN3A, brain-specific homeobox/POU domain protein 3A; DAPI, 4′, 6-diamidino-2-phenylindole; GCL, ganglion cell layer; HPβCD, 2-hydroxypropyl-beta-cyclodextrin; ONC, optic nerve crush.