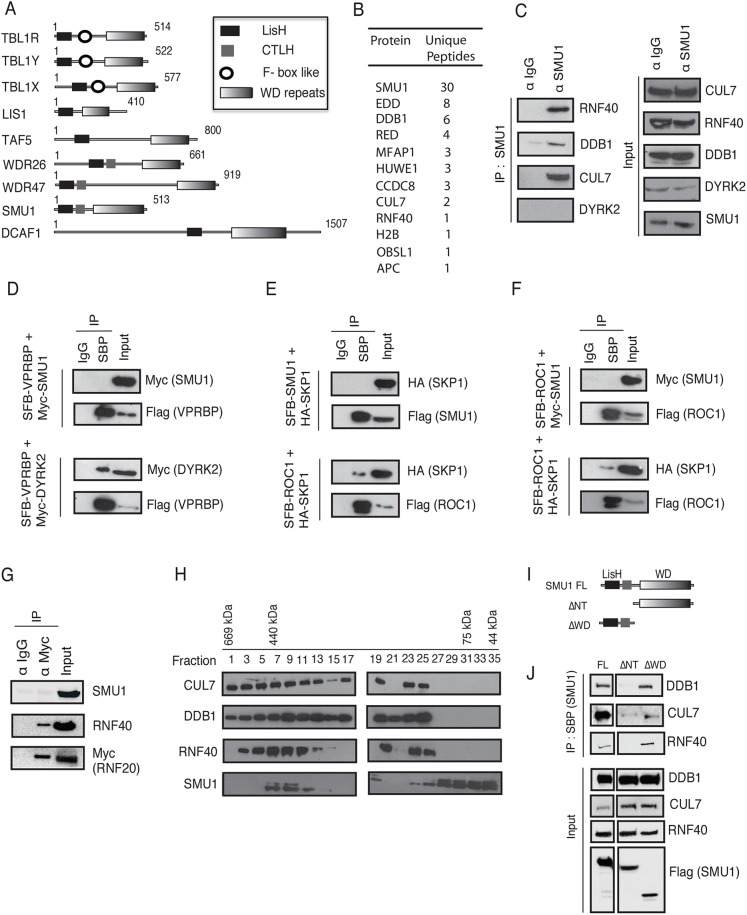
Fig. 1.
SMU1 assembles CRL type E3 ligase complex by interacting with DDB1, CUL7 and RNF40. (A) Proteins that contain a LisH domain and WD repeats. (B) Partial list of SMU1-associated proteins identified by biochemical purification followed by MS analysis were listed together with the number of peptides for each protein. (C) Immunoprecipitation (IP) with control IgG or anti-SMU1 antibody was performed with extracts prepared from HEK-293T cells. The presence of RNF40, DDB1, CUL7 and DYRK2 in these immunoprecipitates was evaluated by immunoblotting with their respective antibodies. (D) SFB-tagged VPRBP, together with either Myc-tagged SMU1 or Myc-tagged DYRK2, was expressed in cells and the interaction of the respective proteins was detected by immunoblotting with the indicated antibodies after pulling down the complexes with streptavidin Sepharose. (E,F) HA tagged-SKP1 together with either SFB-tagged SMU1 or SFB-tagged ROC1 (E), and SFB-tagged ROC1 together with Myc-tagged SMU1 or HA-tagged SKP1 (F) were expressed in cells and their interaction was detected as described in D. (G) HeLa cells expressing Myc-tagged RNF20 were lysed and immunoprecipitation was carried out using either IgG or anti-Myc antibody. The presence of SMU1 and RNF40 was detected in these immunoprecipitates by immunoblotting using specific antibodies. (H) HEK-293T cell extracts were analysed by size-exclusion chromatography using a Sephacryl 300 column. Proteins eluted from the different fractions were immunoblotted with antibodies against the indicated proteins. (I) Domain architecture of full-length SMU1 (SMU1 FL) and its deletion mutants. (J) SFB-tagged SMU1 FL and SMU1 deletion mutants were transfected in HeLa cells. 24 h post transfection, cells were lysed and pull-down was carried out using Streptavidin-binding peptide (SBP) beads. The presence of DDB1, CUL7 and RNF40 in these precipitates was evaluated by immunoblotting with their respective antibodies.