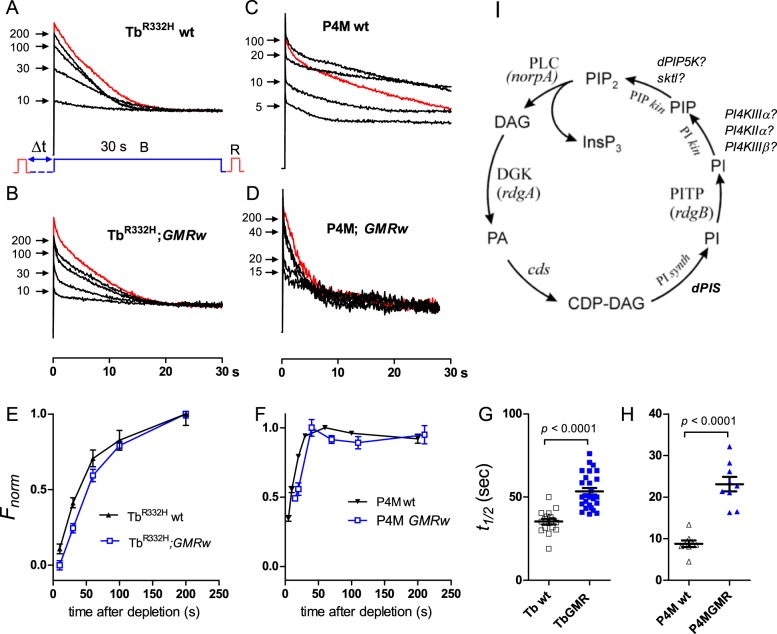
Fig. 1.
PIP2 and PI4P recovery time courses in control flies. (A) TbR332H YFP fluorescence in response to a 30 s blue excitation light measured from rhabdomere patterns in the DPP of otherwise wild-type fly. In a fully dark-adapted fly (red trace) fluorescence decays over ∼20 s as PIP2 is depleted and the TbR332H probe translocates out of the rhabdomere. After M to R reconversion by red (R) light and variable periods in the dark: (Δt=10-200 s), the blue excitation was repeated and the return of the probe to the rhabdomeres (reflecting PIP2 resynthesis) monitored from the instantaneous fluorescence (arrows). (B) Similar protocol from a fly expressing one copy of GMR-Gal4 and UAS-wRNAi (GMRw control for RNAi experiments). (C,D) Similar traces from flies expressing the PI4P-specific P4M-GFP probe on wild-type (C) and GMRw (D) backgrounds. (E,F) Normalised time course of recovery from traces as in A-D after varying times in the dark. (E) PIP2 monitored with TbR332H; mean±s.e.m.; wt, n=17; GMRw, n=27. (F) PI4P monitored with P4M (n=9). (G,H) Time for 50% recovery (t½) for TbR332H (G) and P4M (H) probes (from time course data as in E and F). For both PIP2 (TbR332H) and PI4P (P4M). There were distinct effects on kinetics of depletion and recovery attributable to GMR-Gal4 expression, emphasising the need for GMR-Gal4 controls for UAS-RNAi experiments. (I) The canonical phosphoinositide cycle with identified and candidate genes indicated.