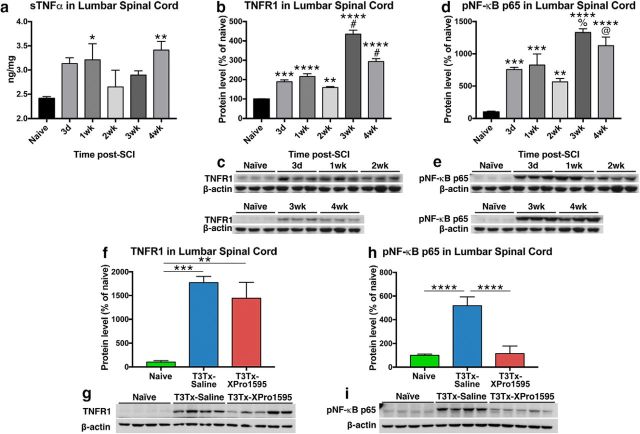
Figure 2.
Intrathecal administration of XPro1595 diminishes signaling downstream of persistent sTNFα/TNFR1 expression below a high-thoracic SCI. a, Levels of TNFα protein in lumbar spinal cord were assessed via ELISA in naive rats and injured rats at different after T3Tx time points (3 d, 4 weeks; n = 4 or 5 per group). Even 1 month after injury, sTNFα levels well below a T3Tx were significantly higher than in naive lumbar spinal cord. b–e, An expression time course of TNFR1 or the phosphorylated (i.e. activated) p65 subunit of NF-κB (pNF-κB p65), a downstream effector of TNFR1 activation, in lumbar spinal cord of uninjured rats or T3Tx rats treated with saline, was determined via Western blot (n = 3 per group). Levels of TNFR1 (b, c) and pNF-κB p65 (d, e) well below a T3Tx were significantly higher than that observed in naive controls, regardless of time point, after SCI. Additionally, there was a significant spike in both TNFR1 and pNF-kB p65 levels between 2 and 3 weeks after T3Tx. f–i, Expression levels of TNFR1 and pNF-κB p65 in lumbar spinal cord of naive rats or T3Tx rats treated with saline or the sTNFα biologic XPro1595 4 weeks after SCI were determined via Western blot (n = 4 or 5 per group). Levels of TNFR1 well below a T3Tx were significantly higher than that observed in naive controls, regardless of whether saline or XPro1595 was infused (f, g). However, despite similarities in TNFR1 expression, inhibiting sTNFα signaling with XPro1595 significantly diminished levels of pNF-κB p65, compared with T3Tx-saline animals. Levels in T3Tx-XPro1595 animals were indistinguishable from that observed in naive animals (h, i). Data are mean ± SEM. p values were determined by a Student's t test or one-way ANOVA and post hoc Fisher's LSD tests: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, #p < 0.0001 versus 2 weeks. %p < 0.001 versus 2 weeks. @p < 0.01.