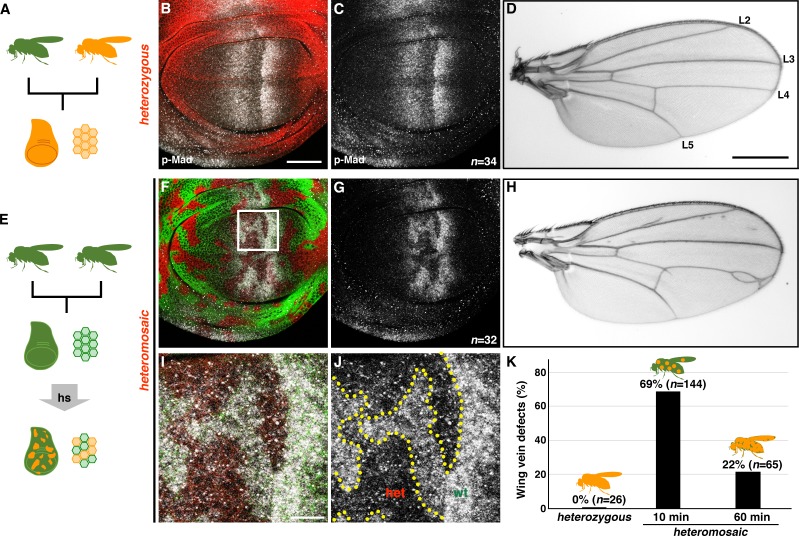
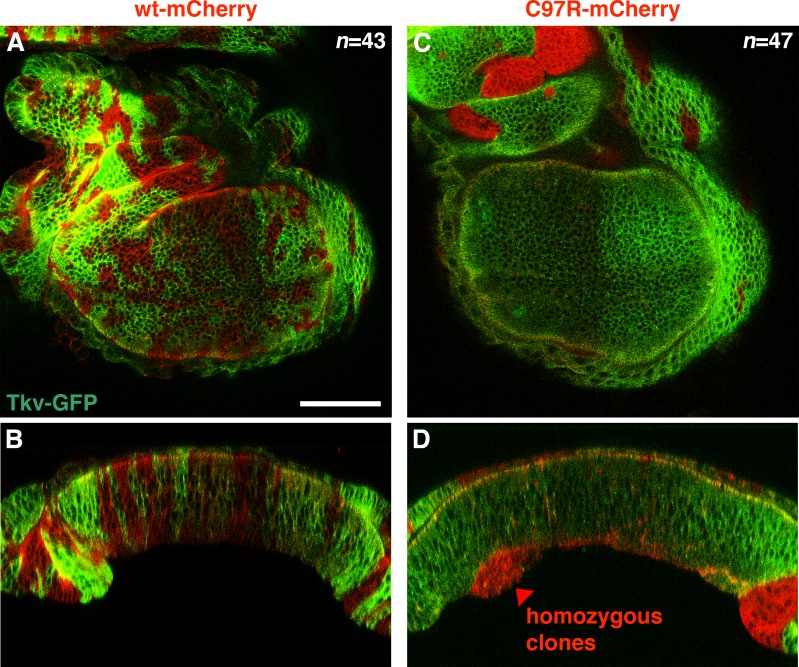
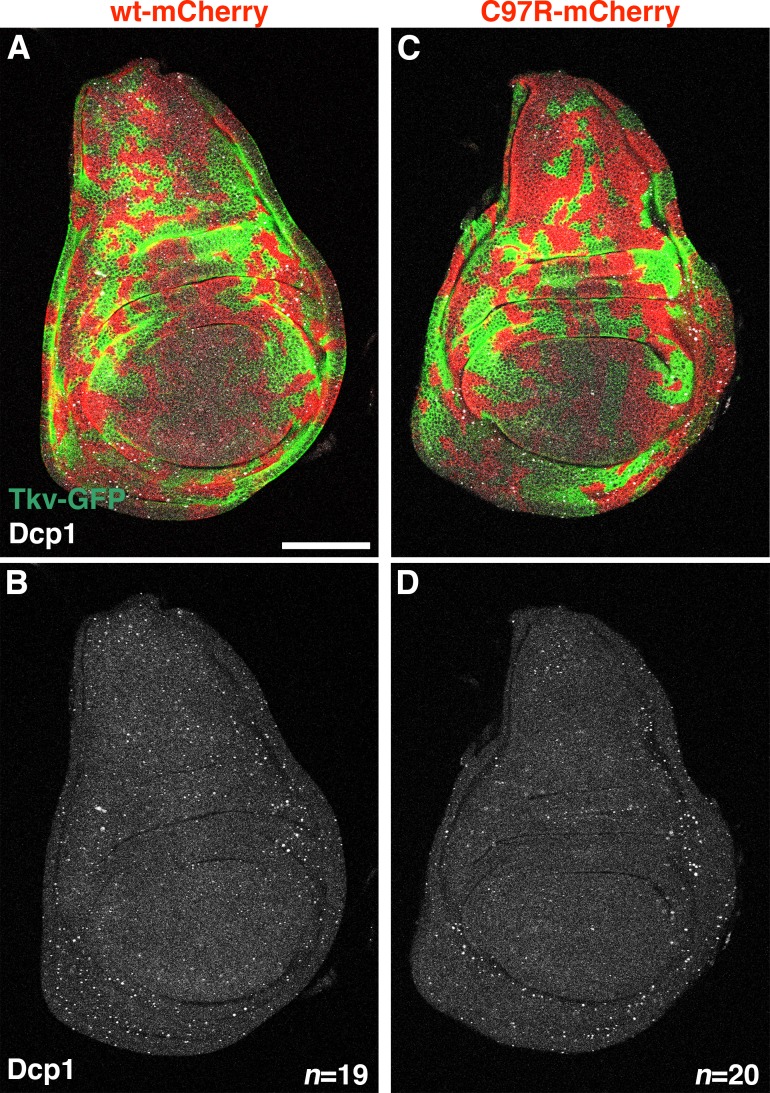
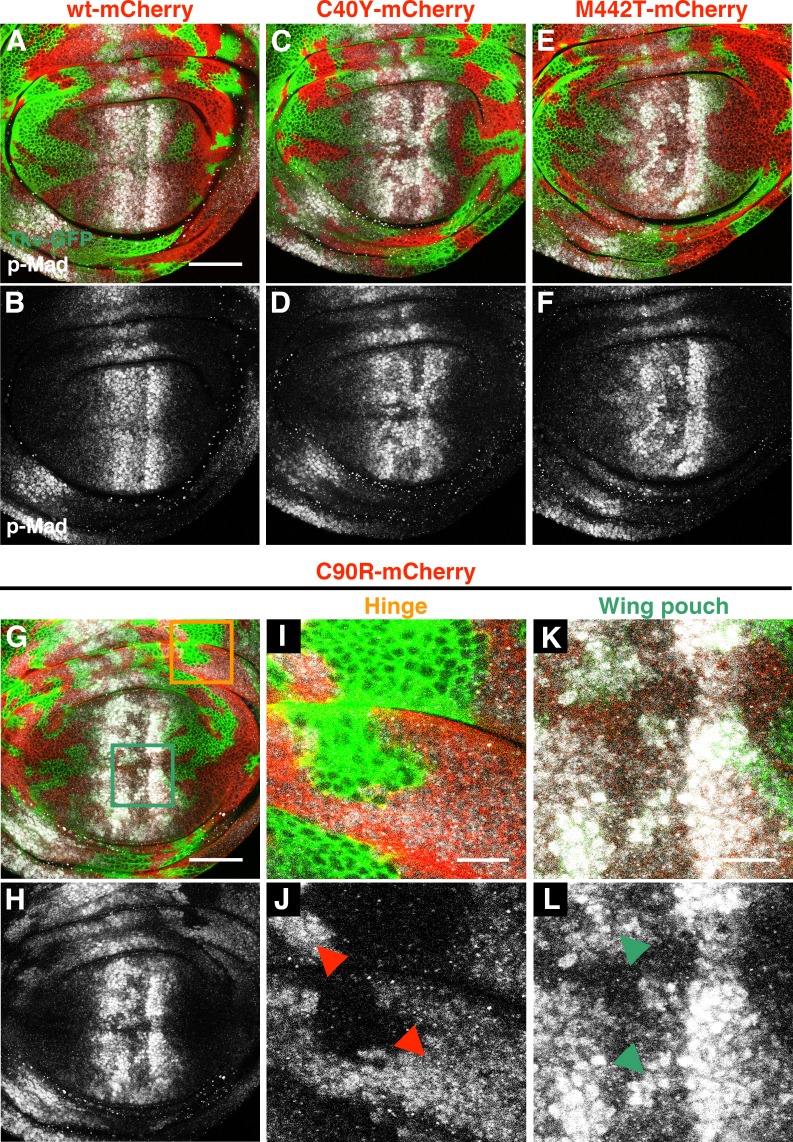
Figure 3. tkv heterozygous mosaicism disrupts wing pattern formation.
(A–D) Germline inheritance of the recessive tkvC97R-mCherry mutation (A). TkvC97R-mCherry (red) and p-Mad (grey) expression in a heterozygous wing disc (B, C). tkvC97R heterozygotes developed normal wings (D). Positions of longitudinal vein L2-L5 are shown. (E–J) Generating somatic clones of tkvC97R heterozygous cells by heat shock (E). wild-type and tkvC97 heterozygous cells are shown by green and red, respectively (F, G, I, J). tkvC97R-mCherry heteromosaicism disrupted the p-Mad activity gradient (grey) and caused wing vein patterning defects (H). (I, J) Magnified images of the boxed area in (F). A similar effect on p-Mad activity was observed in male wing discs, although adult males exhibited milder wing phenotypes. Scale bars: 50 μm for (B), 0.5 mm for (D), and 10 μm for (I). Anterior is oriented to the left side of wing disc images and to the top side of adult wing pictures. (K) Quantification of adult wing phenotypes in tkvC97R-mCherry heterozygous and heteromosaic animals. Longer heat shock generated more uniformly heterozygous cell populations and reduced mosaicism, rescuing wing vein phenotypes.