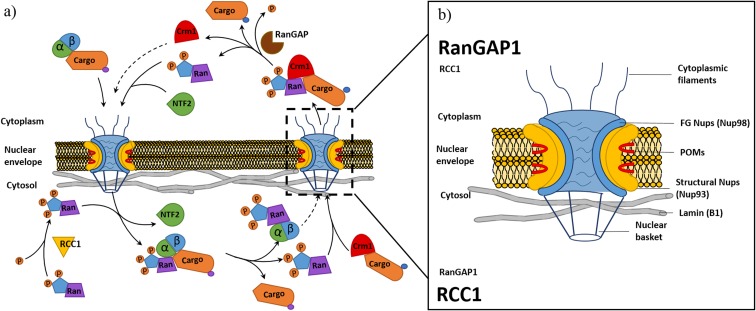
Fig. 1.
Nucleocytoplasmic transport and nuclear pore complex structure: Schematic drawing of classical nuclear import and export processes involved in nucleocytoplasmic transport (a). Nuclear import involves importin-β binding cargo with a nuclear localisation tag (NLS) through the adapter protein importin-α before facilitating import though interactions with nucleoporins (Nups) within the nuclear pore complex (NPC). Upon entry to the nucleus, the Ran GTPase (Ran) bound with guanine triphosphate (RanGTP) binds to the importin complex initiating dissociation of the cargo and return of the import complex to the cytoplasm. The export process involves exportin, chromosome region maintenance 1 (Crm1) binding cargo tagged with a nuclear exiting signal (NES). Crm1 bound to a cargo forms a complex with RanGTP present within the nucleus before export through interaction with Nups within the NPC. Once in the cytoplasm, the cargo is released through hydrolysis of RanGTP to RanGDP by the Ran GTPase activating protein (RanGAP). To maintain the RanGTP/GDP gradient (b), RanGDP is relocated back to the nucleus by nuclear transport factor 2 (NTF2). Once in the nucleus, Ran undergoes a conformational change in response to the regulator of chromosome condensation 1 (RCC1) within the nucleus, stimulating the re-synthesis of RanGTP. The schematic (b) shows the structural arrangement of NPC and nuclear envelope (Lamin), identifying the subcomplexes of the NPC including cytoplasmic filaments, FG Nups, structural Nups, POMs and nuclear basket Nups