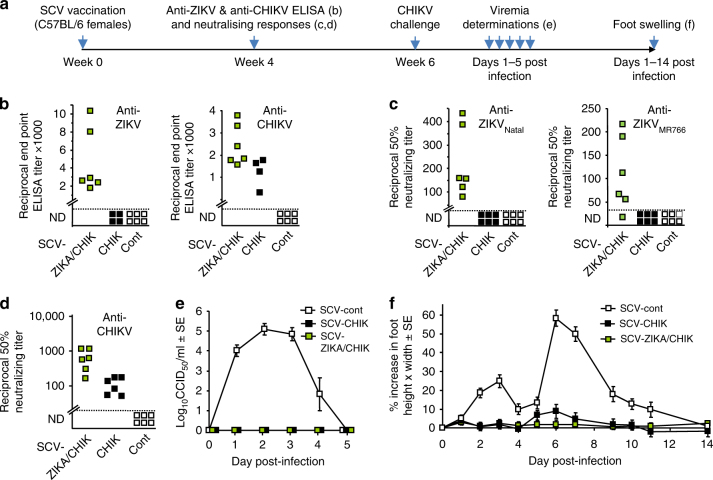
Fig.2.
ZIKV and CHIKV antibody responses in C57BL/6 mice and CHIKV challenge. a Timeline of vaccination, antibody assays, CHIKV challenge, viremia, and foot measurements. b End point IgG ELISA titers against ZIKV and CHIKV 4 weeks post vaccination with 106 pfu of the indicated SCV vaccine; SCV-ZIKA/CHIK, SCV-CHIK, or SCV-cont. The limit of detection was 1 in 30, meaning that a 1 in 30 dilution of sera was the highest (starting) concentration of sera used in the assay. ND not detected. (n = 6, except for SCV-CHIK n = 4 mice per group). SCV-ZIKA/CHIK vaccinated mice had significantly higher CHIKV and ZIKV titers than SCV-cont vaccinated mice (both p = 0.005, Kolmogorov–Smirnov tests). (Differences in anti-CHIKV titers between SCV-CHIK and SCV-ZIKA/CHIK were not significant). c Neutralizing titers against ZIKVNatal and ZIKVMR766 in mice vaccinated with the indicated SCV vaccines (n = 6 per group). Limit of detection 1 in 30. (p = 0.005 and 0.031, Kolmogorov–Smirnov tests). d Neutralizing titers against CHIKV. Limit of detection 1 in 30. (Compared with SCV-cont both p = 0.005, Kolmogorov–Smirnov tests). e Viremia of mice described in d, after challenge with CHIKV (6 weeks post vaccination). Limit of detection 2 log10CCID50/ml. For days 1–3 the viremia in SCV-ZIKA/CHIK vaccinated mice was significantly lower than in SCV-control vaccinated mice; all p = 0.005, Kolmogorov–Smirnov tests. f Foot swelling of mice described in e. From days 2–10 the foot swelling in SCV-ZIKA/CHIK vaccinated mice was significantly lower than in SCV-control vaccinated mice; p = 0.03–0.001, Mann–Whitney U tests. Error bars represent standard error of the mean