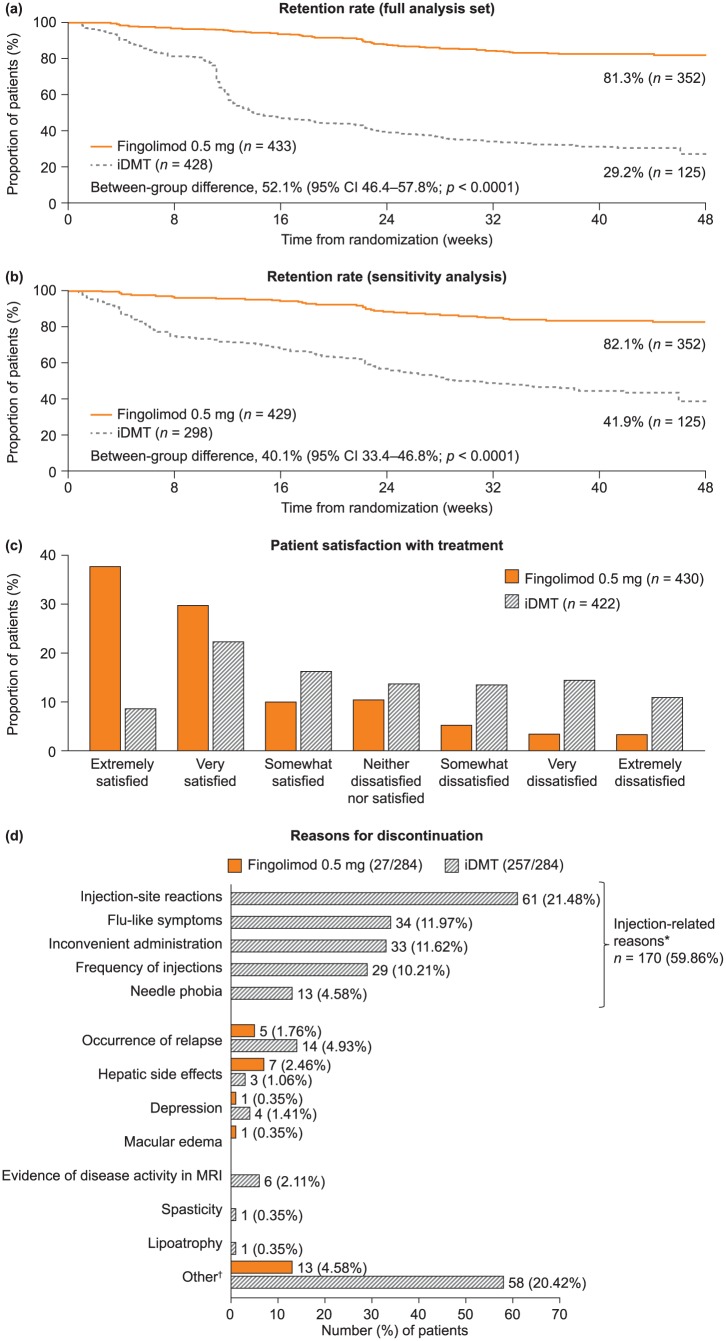
Figure 2.
Retention rates, patient satisfaction with treatment, and reasons for discontinuing randomized treatment in PREFERMS. (a) The primary outcome of retention rate in the two treatment groups as a Kaplan–Meier plot, statistically analyzed as a log-rank test adjusted for treatment. The data were also analyzed by a Cochran–Mantel–Haenszel test, a logistic regression and a Cox proportional hazard model, which were adjusted for treatment and treatment naïvety. The data were also measured by normal approximation performed using continuity correction. ****p < 0.0001 for all analyses. (b) Sensitivity analysis of the retention rate shown in panel (a) excluding patients who switched treatment for nonefficacy or safety reasons before week 12 in violation of the study protocol, and all patients switching treatment between days 77 and 110 in case any had enrolled with the intention of switching from iDMT to fingolimod as soon as it became permissible for any reason. (c) Patient-reported satisfaction as measured by the Medication Satisfaction Questionnaire at last assessment. The overall difference across categories between fingolimod 0.5 mg and iDMTs is significant (Cochran–Mantel–Haenszel test using modified ridit scores adjusted for treatment and treatment naïvety; p < 0.0001). (d) Primary reasons for discontinuing randomized treatment. Labels for each bar represent the number (percentage of the total number of patients discontinuing randomized treatment). *Injection-related reasons for discontinuation are listed on the left of the corresponding area of the graph. †Reasons for ‘Other’ stated in the fingolimod group include the following [n = 1 (0.35%), unless stated otherwise]: abnormal platelet counts; adverse drug reaction; AE of headache; AE of joint pain; AE of papilloma, hair loss, herpes simplex virus, and lymphopenia; anxiety; hypertension; lymphopenia [n = 2 (0.70%)]; mouth sores; possible diagnosis of macular edema; side effects; and tolerability. Reasons for ‘Other’ in the iDMT group include the following [n = 1 (0.35%), unless stated otherwise]: abdominal pain, chest pain, flushing; AE; AE of bilateral lower extremity edema; AE of headache; allergic reaction [n = 2 (0.70%)]; anxiety; anxiety and depression; anxiety and fatigue; arthralgia and myalgia; bruising; convenience; fatigue [n = 2 (0.70%)]; flu-like symptoms [n = 2 (0.70%)]; general body ache [n = 2 (0.70%)]; headache [n = 2 (0.70%)]; injection fatigue; injection pain [n = 8 (2.82%)]; injection reaction; intolerant; lack of efficacy; muscle aches; mood altering; needle phobia [n = 4 (1.41%)]; palpitations; panic and irritability; patient choice [n = 10 (3.52%)]; possible seizure exacerbation; rash; relapse [n = 3 (1.06%)]; stopped taking medication; and treatment dissatisfaction [n = 2 (0.70%)]. AE, adverse event; CI, confidence interval; iDMT, injectable disease-modifying treatment; MRI, magnetic resonance imaging; PREFERMS, Prospective, Randomized, active-controlled, open-label study to Evaluate patient retention on Fingolimod versus approved first-line disease modifying thErapies in adults with Relapsing remitting Multiple Sclerosis.