Figure 1.
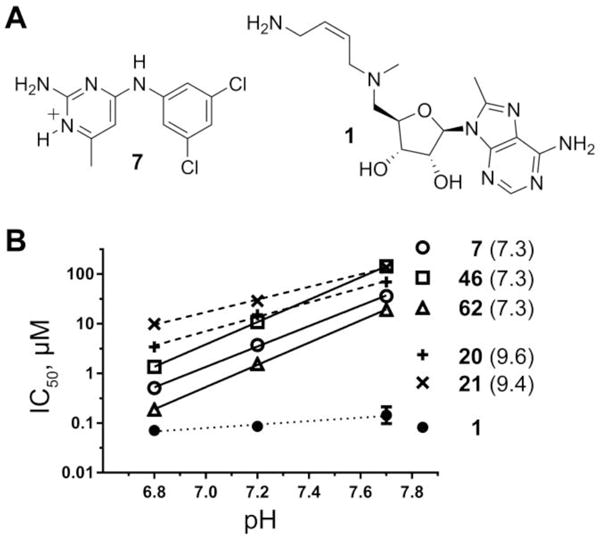
Pyrimidineamines are pH-dependent TbAdoMetDC inhibitors. (A) Structure of the protonated form of pyrimidineamine 7 (N1 is predicted to have pKa of 7.3), and structure of mechanism-based inhibitor Genz-644131 (1). (B) IC50 of pyrimidineamines plotted as a function of the assay buffer pH and fitted with linear regression analysis in Prism (GraphPad). Predicted pKa(N1) values are shown in parentheses. The slopes for pyrimidineamines with pKa(N1) = 7.3 (solid lines) are compared with those for analogs with pKa(N1) > 9 (dashed lines), and with that for a non-pyrimidineamine control (dotted line).
