Table 4.
SAR of meta aniline ring substitutions
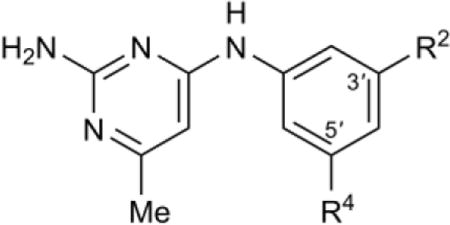
| |||||
|---|---|---|---|---|---|
| Compound | R2 | R4 | Tb IC50,b μM | Hs IC50,c μM | Tb427 EC50,d μM |
| 7 | Cl | Cl | 3.7 (3.3–4.1) | 27% at 180 | 5.6 (4.5–7.1) |
| 32 | Br | Cl | 2.2 (2.0–2.4) | 33% at 180 | 4.7 (3.9–5.8) |
| 33 | CN | Cl | 3.9 (3.2–4.8) | 29% at 180 | 12 (10–14) |
| 34 | CF3 | Cl | 3.9 (3.4–4.4) | 39% at 180 | 13 (11–15) |
| 35 | Ph | Cl | 3.3 (2.8–3.9) | 30% at 63 | 4.5 (2.1–9.4) |
| 36 | SF5 | Cl | 16 (14–18) | >180 | 13 (11–15) |
| 37 | SF5 | Br | 8.7 (7.4–10) | >180 | 0.45 (0.32–0.62) |
| 38 | Et | Br | 7.6 (5.7–10) | >180 | 5.5 (4.6–6.6) |
| 39 | OEt | Br | 6.0 (5.2–6.9) | 36% at 180 | 4.4 (4.2–4.7) |
| 40 | OCH2CF3 | Br | 1.6 (1.1–2.4) | 44 | 4.2 (3.6–4.8) |
| 41 | OCH2CHCH2 | Br | 4.5 (4.1–4.9) | 28% at 180 | 4.4 (3.7–5.3) |
| 42 | OBu | Br | 4.5 (4.0–5.1) | >180 | 1.9 (1.7–2.1) |
| 43 | OCH2iPr | Br | 11 (9.5–12) | >180 | 4.4 (3.5–5.5) |
| 44 | Br | Br | 1.9 (1.7–2.1) | 31% at 180 | 4.8 (4.1–5.6) |
| 45 | SO2Me | SO2Me | 1.5 (1.3–1.7) | 39% at 180 | >100 |
| 46 | CF3 | CF3 | 11 (9.8–12) | >180 | 14 (11–17) |
| 47 | OMe | OMe | 17 (14–20) | 21% at 180 | 41 (33–51) |
| 48 | CO2H | CO2H | >180 | >180 | >100 |
| 49 | CO2Me | CO2Me | 40% at 180 | >180 | 42 (22–78) |
| 50 | Ph | Ph | >180 | >180 | 1.5 (1.3–1.6) |
| 51 | N-(3,5-Cl2Ph)−acetamide | N-(3,5-Cl2Ph)−acetamide | >180 | >180 | 2.0 (1.2–3.7) |
| 52 | Me | Me | 141 (119–167) | >180 | 19 (17–22) |
| 53 | tBu | tBu | >63 | >63 | 4.1 (4.1–4.5) |
| 54 | SF5 | H | 23% at 180 | >180 | 14 (11–17) |
| 55 | Cl | H | 35% at 180 | >180 | 42 (34–52) |
| 56 | Br | H | 41% at 180 | >180 | 22 (19–26) |
| 57 | F | H | >180 | >180 | >25 |
| 58 | SMe | H | 149 (122–181) | 23% at 180 | >25 |
| 59 | H | H | >180 | >180 | >25 |
| 60 | F | F | 180 (160–201) | >180 | 70 (61–80) |
| 61 | F | Cl | 32 (13–82) | >180 | 17 (15–20) |
All compounds have pKa(N1)a of 7.3.
See footnotes for Table 1.
