Table 5.
SAR of ortho- and para-aniline ring substitutions
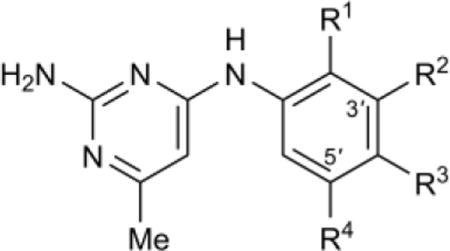
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | Tb IC50,b μM | Hs IC50,c μM | Tb427 EC50,d μM |
| 7 | H | Cl | H | Cl | 3.7 (3.3–4.1) | 27% at 180 | 5.6 (4.5–7.1) |
| 62 | H | Cl | F | Cl | 1.5 (1.4–1.8) | 160 (135–191) | 4.8 (3.3–7) |
| 63 | H | Br | F | Br | 1.6 (1.3–1.8) | 43% at 180 | 4.6 (3.6–5.9) |
| 64 | H | Cl | Cl | Cl | 11 (8.8–13) | >63 | 4.5 (3.4–5.9) |
| 65 | H | Cl | Me | Cl | 20 (17–24) | >63 | 4.4 (4.2–4.8) |
| 66 | H | Cl | OPh | Cl | 38% at 180 | >180 | 1.1 (0.97–1.3) |
| 67 | H | Cl | OH | Cl | 33% at 180 | >180 | 13 (10–16) |
| 68 | H | Cl | OCHF2 | Cl | >63 | >63 | 4.5 (3.7–5.4) |
| 69 | H | Cl | NMe2 | Cl | >180 | >180 | 5.8 (4.2–7.9) |
| 70 | H | Cl | N-morpholino | Cl | >180 | >180 | 18 (14–24) |
| 71 | H | Cl | N-piperidino | Cl | >180 | >180 | 2.1 (1.3–3.2) |
| 72 | OH | Cl | H | Cl | 37 (29–46) | >180 | 26% at 100 |
| 73 | F | Cl | F | Cl | 2.2 (2.0–2.6) | >63 | 10 (8.5–12) |
| 74 | Cl | H | H | Cl | 115 (89–150) | >180 | 23% at 25 |
| 75 | Cl | Cl | H | H | 137 (95–198) | >180 | 27% at 25 |
| 76 | Cl | H | Cl | H | >180 | >180 | 28% at 25 |
| 77 | Me | H | H | H | >180 | >180 | >25 |
| 78 | SMe | H | H | H | >180 | >180 | >25 |
| 79 | Br | H | H | H | >180 | >180 | >25 |
| 80 | Me | Cl | H | H | >180 | >180 | 29% at 25 |
| 81 | Me | H | H | Cl | >180 | >180 | 28% at 25 |
| 82 | Me | H | Cl | H | >180 | >180 | >25 |
| 83 | F | H | Br | H | >180 | >180 | 25% at 25 |
| 84 | Cl | H | H | CF3 | 199 (164–240) | >180 | 28% at 25 |
| 85 | H | H | Cl | H | >180 | >180 | 28% at 25 |
| 86 | H | H | Et | H | >180 | >180 | 19 (17–22) |
| 87 | H | H | iPr | H | >180 | >180 | 11 (8.5–15) |
| 88 | H | H | CF3 | H | >180 | >180 | 15 (13–17) |
| 89 | H | H | 3,5-Cl2Ph | H | >180 | >180 | 1.0 (0.88–1.2) |
| 90 | H | F | F | H | 33% at 180 | >180 | 25% at 25 |
All compounds have pKa(N1)a of 7.3 except 67 (pKa(N1)= 7.0); 72 (pKa(N1)= 7.1); 73, 74, and 83 (pKa(N1)= 7.2).
See footnotes for Table 1.
