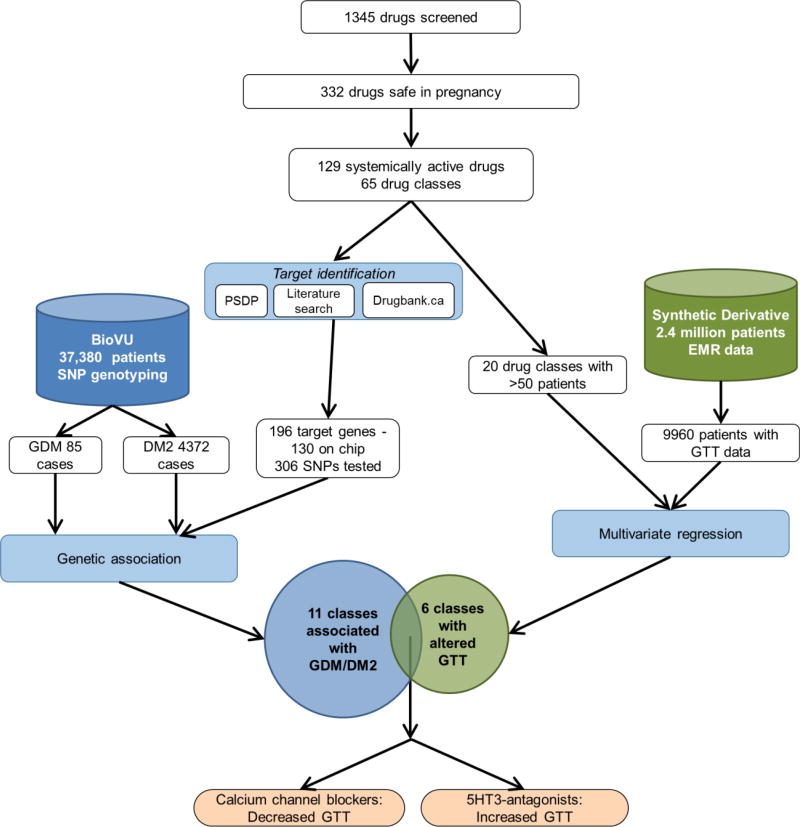
Figure 1. Experimental design.
We screened 1345 drugs and identified 332 considered safe in pregnancy. Of these, 129 drugs in 65 classes are systemically active, have identifiable human targets, and are not contraindicated by the US FDA. We pursued a two-pronged strategy. In the first (left side), we identified the drug target genes using publicly available data. Of the 196 target genes, 130 genes, represented by 306 SNPs, were present on our genotyping platform. We used SNP genotyping data from 37,380 patients with ICD9-level diagnostic information (BioVU). We examined the association between the genes targeted by our candidate drugs and GDM or DM2, combining our analyses using gene set analysis, which resulted in 11 drug classes genetically associated with GDM/DM2. In the second strategy (right side), we generated a cohort of patients with GTT data and examined the effect of drug exposure on GTT values in a multivariate regression. We identified 6 classes of drugs associated with changes in GTT. The overlap of these two independent strategies (bottom) are two drugs – 5HT-3 antagonists, which are associated with increased GTT, indicating worse glucose tolerance, and calcium channel blockers, which are associated with decreased GTT, indicating improved glucose tolerance.