Figure 7. Factors contributing to mitochondrial dysfunction in diabetic nephropathy.
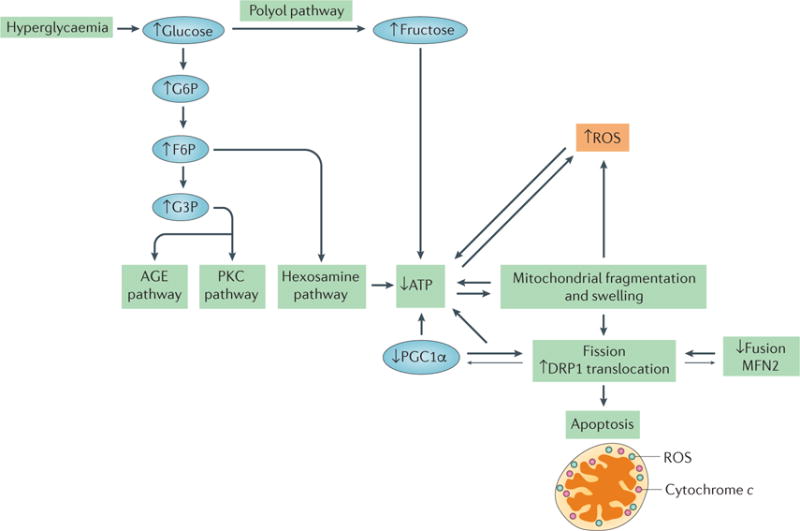
Hyperglycaemia is the primary contributing factor to mitochondrial dysfunction in diabetic nephropathy. An increase in glucose level results in an increase in glycolysis, in turn activating the advanced glycation end product (AGE) pathway, the protein kinase C (PKC) pathway and the hexosamine pathway, which results in a decrease in ATP levels. Hyperglycaemia also activates the polyol pathway, which increases fructose levels and, consequently, decreases ATP levels. Mitochondrial fragmentation and swelling is observed in early diabetic nephropathy, leading to an increase in fission and the production of reactive oxygen species (ROS). The correlations between increased mitochondrial fragmentation and decreased ATP, and between ROS production and decreased ATP, are interdependent. Whether one causes the other is unclear, as depicted by the bidirectional arrows. Decreases in the levels of mitofusin 2 (MFN2) and peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α) correlate with, and might contribute to, the increase in mitochondrial fission observed in diabetic nephropathy, as indicated by the larger arrows pointing towards increased mitochondrial fission. Decreases in mitochondrial energetics that are caused by changes in mitochondrial morphology and hyperglycaemia lead to apoptosis in diabetic nephropathy. F6P, fructose-6-phosphate; G6P, glucose-6-phosphate; G3P, glyceraldehyde-3-phosphate.
