Figure 3.
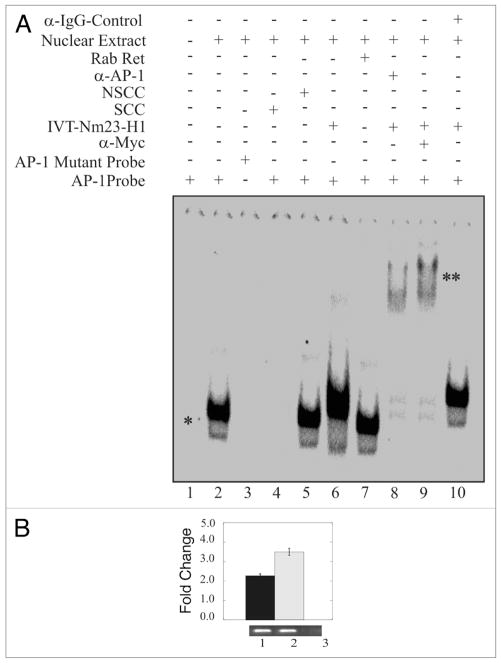
Nm23-H1 forms a complex with the AP1 transcription factor bound to its binding site. EMSA showed a specific AP1 shift observed when nuclear extract was added to reaction with labeled AP1 specific probe. The specificity of this shift was verified through the disappearance of the shift in the presence of mutant AP1 probe or specific cold competitor (A and compare lanes 1, 2 with 3 or 4). The shift was not disrupted when we used a non-specific cold competitor (lane 5). The mobility of the AP1 probe was reduced by the presence of in vitro translated Nm23-H1 (lane 6). There was no apparent change on effect when we used un-programmed the rabbit reticulocytes (lane 7). The presence of Nm23-H1 in the complex was verified by additional supershifting in the presence of anti-myc antibody (1 μg) used to detect Nm23-H1 (lane 8). The presence of AP1 was shown by AP1-specific supershift (lane 9). Additionally, no specific supershift was observed with when the IgG control antibody was used (lane 10). (B) Chromatin from Nm23-H1 overexpressing cells was cross-linked with 1% formaldehyde followed by washing with PBS. Cell nuclei were released from these cells followed by sonication of chromatin to approximately 700 bp. Sonicated chromatin were diluted with chromatin immunoprecipitation dilution buffer. Chromatin was immunoprecipitated using antibodies specific for Nm23-H1. The primer sets mentioned in material and methods were used to amplify the regions 100 bp sequence on CyD1 promoter containing the AP1 cognate sequence. Amplified bands were quantitated and the relative amounts were calculated. Lane 1, DNA purified from 10% of the total chromatin; lane 2, DNA from chromatin immunoprecipitated with rabbit polyclonal anti-Nm23-H1; lane 3, DNA from chromatin immunoprecipitated with control immunoglobulin G antibody (Sigma, Inc.).