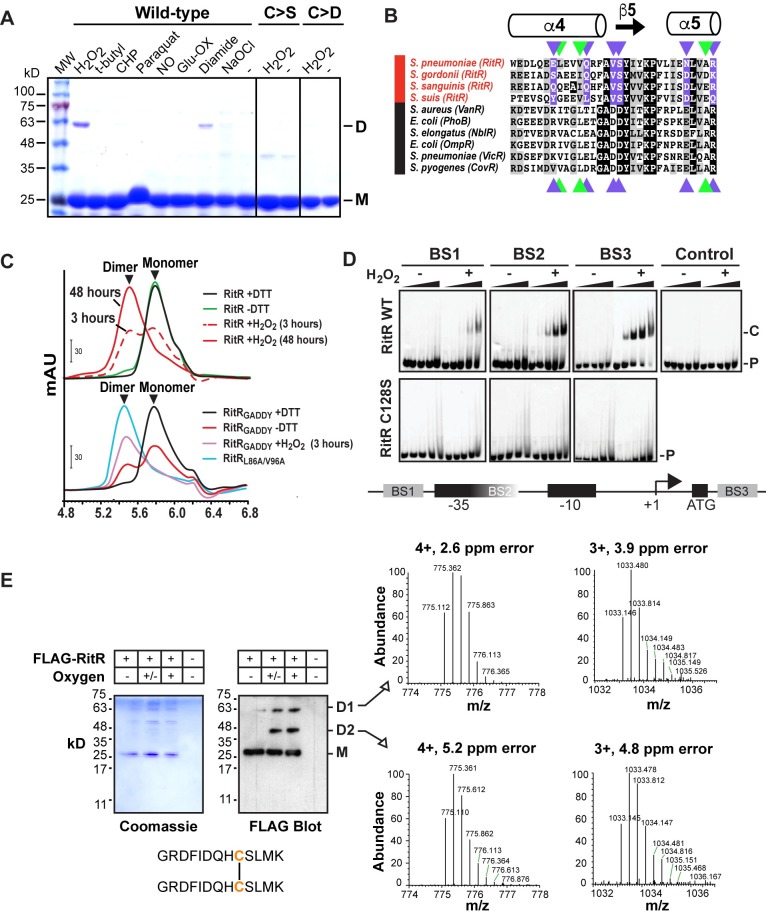
Fig 2. RitR has evolved to dimerize through Cys128-mediated oxidation by H2O2.
(A) Non-denaturing SDS-PAGE gels of RitR WT, C>S and C>D mutants plus various oxidants in the presence of 3 mM DTT. D, dimeric RitR; M, monomeric RitR. MW, Molecular Weight ladder; H2O2, hydrogen peroxide; t-butyl, tert-butyl hydroperoxide; CHP, cumene hydroperoxide; NO, nitrous oxide; Ox-Glu, oxidized glutathione; NaOCl, sodium hypochlorite. (B) Alignment of REC/ALR α4-β5-α5 dimerization domains [30] from RitR homologs (red) and canonical REC domain sequences (black). The black boxes are identical residues and the grey boxes are similar residues. Residues colored purple and green represent key charged and hydrophobic residues, respectively, involved in typical REC dimerization. Note that several of these key residues are changed in RitR homologs. (C) SEC of wild-type RitR with (+) or without (-) addition of DTT or H2O2 (top graph), or RitR changed back to the canonical GADDY sequence (RitRGADDY; bottom graph). For comparison, the RitRL86A/V96A mutant is shown, which naturally dimerizes without addition of oxidant [30]. mAU; milli Absorbance Units. (D) EMSAs of RitR wild-type (WT) and the C128S mutant in the presence (+) and absence (-) of H2O2. RitR proteins were added at 0, 0.22, 0.66, 2.2 and 6.6 μM concentrations (left to right) in the presence of hexofluorescein (HEX)-labeled BS1-3 double-stranded DNA oligomers. A HEX labeled control oligo was also used. P, Hex DNA probe; C, RitR-DNA shifted complex. Below is a schematic diagram of the Piu promoter and regulatory region showing the location of RitR binding sites 1–3 (BS1-3) as previously described [25]. (E) Mass spectrometry (MS) analysis of the Cys128 disulfide bridge formation in vivo. Upper (D1), middle (D2) and lower bands (M) of RitR as identified from the anti-FLAG western blot and accompanying Coomassie stain were excised from the gel and determined to contain RitR using MS. (+) oxygen = cells were aerated, (+/-) oxygen = cells were grown statically in 5% CO2, and (-) oxygen = cells were grown anaerobically before addition of IAA and RitR immunoprecipitation. The MS identified Cys128 linked peptide is shown with Cys128 colored in orange. Data shown in A-E are representative of at least two independent experiments.