Abstract
Objective:
The survival rate of children treated for cancer is currently about 80% at 5 years and we estimate that about 50,000 adults in France have survived childhood cancer. In 2011, there was a call for projects relating to long-term follow-up (LTFU), which led to several studies being conducted. Five years later, we sent a questionnaire to present LTFU in France and describe its strengths and weaknesses and to establish appropriate steps that should be taken.
Methods:
A questionnaire was sent by email to all the members of the French Society of Childhood Cancers in spring 2016. The study involved 44 centres/hospitals with a Paediatric Oncology Department.
Results:
54 answers were analysed, provided by 31/44 (70%) centres working together with the French Society of Childhood Cancers. Screening is the main objective of LTFU care (90%). The main difficulties that arose were: lack of sufficient time to devote to this activity (57%), difficulties contacting adult childhood cancer survivors (aCCSs) (26%), aCCSs who ultimately did not show up to the consultation (19%), cost (15%), and lack of organization (13%). Seven LTFU programmes were identified: two regional organizations (Rhône Alpes and Grand Ouest), four centre-size organizations, and one national study (involving 15 Haematology Centres) relating to Child and Adolescent Leukaemia.
Conclusion:
LTFU is a major concern for French centres specialized in paediatric oncology. Organization is not well defined and difficulties still arise (Who are the best care providers? What frequency of care is most appropriate? etc.).
Advances in knowledge:
LTFU focused on health problems (physical, psychological, social, economic issues) that affect CCSs is needed to ensure that these patients regain the most optimal physical and emotional health possible. Practitioners suggest different ways to improve LTFU, such as national co-operation with Epidemiology Registers to promote homogenous LTFU care.
Introduction
In the 21st century, there are about 50,000 new cases of cancer per year in young people under the age of 25 in the European Union. New chemotherapy regimens combined with modern radiotherapy techniques and optimized supportive care have largely contributed to the significant increase in survival rates, which are currently above 80%.1–4 For adult childhood cancer survivors (aCCSs), several publications described cumulative prevalence rates between 40 and 84% of chronic health problems, which can be disabling and/or life-threatening.5–9 It is, therefore, recommended that survivors attend long-term follow-up (LTFU) care for the prevention, early detection, and treatment of late effects.10–14 Clinicians must balance providing sufficient information with avoiding causing unnecessary anxiety.15–18
Treatment protocols, diagnosis, and patient initial characteristics help identify general risk factors for late side effects in groups, but not individuals.19 There is little research to guide clinicians on how and when to best provide cancer survivors with information relating to late side effects.20 Not all aCCSs are at risk of late side effects and monitoring, recommendations should, therefore, be risk based.21, 22 In the future, this risk should be adapted to genetic polymorphism that could make an individual more susceptible to late sequelae.23
In France, LTFU is a concern for all centres specializing in paediatric oncology and the SFCE (Société Française des Cancers de l’Enfant—French Society for Childhood Cancers), as can be seen by the existence of a multiprofessional committee dedicated to this issue. Several French cohorts and registries already exist and have been described in literature. In 2011, a call for projects relating to this field led to several studies being conducted. Nevertheless, all these actions were not implemented throughout the entire country. Five years later, therefore, we sent a questionnaire to present LTFU in France, describe its strengths and weaknesses, and establish appropriate steps that should be taken.
This article describes the results of the most recent questionnaire, sent out 10 years after the first. It was addressed to all the members of the SFCE and discusses the concerns surrounding LTFU.
Methods and materials
A questionnaire was sent by email to all the members of the SFCE in spring 2016 (i.e. 13 surgeons, 119 paediatric oncologist/haematologists, 8 radiation oncologists). We asked them to answer personally, but it was not obligatory to provide a single answer for the same department. One month later, we sent an email reminder thanking the individuals who had answered. If there was still no response from some members, we wrote one last email to paediatric oncologists. The questionnaire was made up of 3 parts and 30 questions: (a) identification of the physician and definition of LTFU (9 questions); (b) description of the traditional follow up just after treatment (2 questions); and (c) LTFU for aCCSs (19 questions). The main objective was to describe LTFU in France in 2016. The secondary objectives were to compare the differences that occurred in the last 10 years, to assess the content and aims of follow-up, to describe problems relating to LTFU, and to establish what steps should be taken ideally to improve LTFU. In the case of respondents running a LTFU clinic, we asked them to describe their organization system and tell us what they think about it and whether it could be expanded throughout the country. We included specific questions about second cancer screening.
All respondents agreed to take part in this study. According to French guidelines on ethical considerations in research involving human participants, a survey on healthcare systems and physicians’ knowledge does not raise any ethical concerns. A formal approval from the Ethics Committee was, therefore, deemed unnecessary.
Descriptive analyses were carried out to summarize the results. To compare with the survey carried out in 2006, analyses were performed with XLSTAT v. 2016, mainly using Pearson’s χ2 test. We took into account the origin of the replies and we differentiated whether these were responses from the same city, the same hospital/centre, or the same department. For the analysis, given the size of Paediatric Oncology departments in “Ile de France”, we considered the districts (arrondissements) of Paris and the neighbouring towns as different cities (n = 5). In the rest of this article, we use the generic word “centre” to refer to hospitals or cancer centres.
Results
Respondents
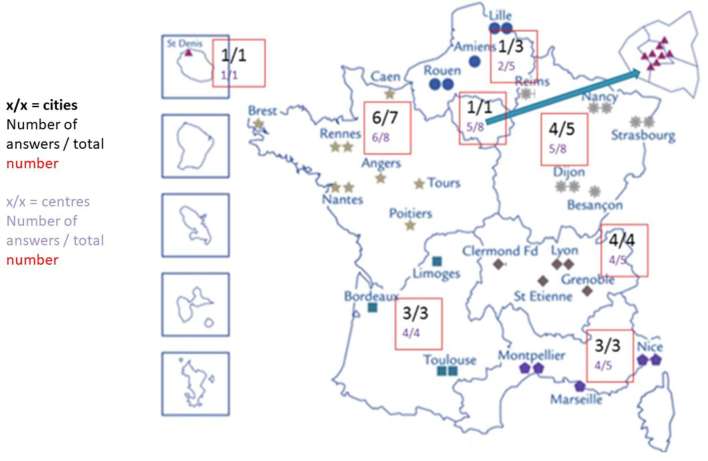
We collected 54 answers from 23 of the 27 (85%) cities that are SFCE members, making up 31/44 (70%) of the centres working with the SFCE (there are more centres than cities due to hospitals and oncology centres) (Figure 1). The physicians were mainly paediatric oncologists (40, 34%), radiation oncologists (6, 75%), and surgeons (6, 46%, including 4 orthopaedic surgeons and 2 digestive surgeons). We collected several answers in 12/23 cities (52%), in 11/31 centres (35%). Two answers specified that it was a co-ordinated answer of all the paediatric oncologists in the department. 90% of respondents were aware of the existence of a specific LTFU committee in the SFCE.
Figure 1.
Map of centres working with the SFCE (involved in paediatric oncology) showing the distribution of the answers to the survey. SFCE, French Society for Childhood Cancers.
Definition of long-term follow-up
The results are various: 45% answered that LTFU starts at the end of the treatment, 5% indicated it begins 1 year after the end of the treatment, 43% specified it starts after 5 years without any treatment, and 7% answered that it depends on the disease. Moreover, 11% added that LTFU concerns children who became adults. The answers were discordant in 10 centres of the 11 in which several physicians provided answers.
98% of the physicians were aware of the risks arising in the long term (well: 79%; approximately: 19%), and 83% were of the opinion that they can oversee the LTFU, of which 33% were not directly involved in the centre.
One of the first questions was asked as an open question, and aimed to define the objectives of LTFU. Screening was the main answer (90%) and 35% also raised the problem of second cancers. The other objectives were related to various areas: fertility (14%), puberty/growth (7%), social and psychological aspects (18%), genetic aspect (12%), family care (7%), and late relapse (7%). LTFU programmes should organize care (64%), (re)inform patients about their disease and the treatment they received (18%), and educate CCSs about what they can/should do to improve their quality of life. LTFU programmes should be developed to learn more about LTFU sequelae and to study how the health costs for aCCSs can be decreased after treatment.
Follow-up after treatment
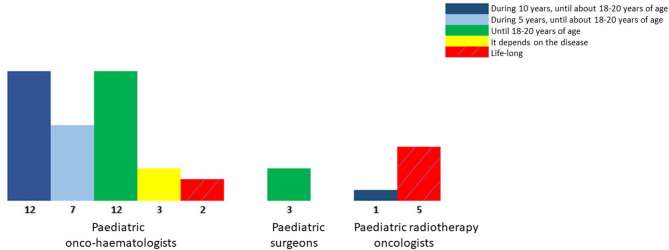
The follow-up implemented after treatment is mainly homogenous, with 93% of the physicians answering that they follow up patients at least until they reach 18 years of age, of which 16% follow up patients during their entire life if the aCCS agrees to this. “Life-long follow-up” is mainly provided by radiation oncologists (5/6) (Figure 2).
Figure 2.
Usual follow-up done in France after treatment, depending on the specialization.
Long-term follow-up for aCCSs
Seven LTFU programmes were identified: two regional organizations (Rhône Alpes and Grand Ouest) and four centre-size organizations.14 In addition, there is the LEA study (Leucémies de l’Enfant et de l’Adolescent, Child and Adolescent Leukaemia), which was started in 2004 and now covers 15 haematology centres.24 Different models, already described in a French article, were tested, including clinical LTFU, follow-up by the general practitioner (GP), and therapeutic education of patients.14 Half of the programmes have dedicated time for LTFU. LTFU involves medical time (83%) [internists (80%), GPs in hospital (40%), paediatric oncologists or adolescent young adult oncologists (100%)] and paramedical time (67%) [psychologists (60%), nurses (40%), adolescent young adult networks (40%), educators (20%), and associations (20%)]. For the other half, the main answer was GPs (52%) or adult oncologists/haematologists (26%) for some patients (history of bone marrow transplant, history of cerebral tumour, patients with a pre-disposition syndrome). Some centres asked how satisfied GPs were with their organization: in three cities where a LTFU programme exists, the GPs were satisfied. In two cities where there is no LTFU programme, the GPs answered that they cannot provide LTFU due to the low percentage of aCCSs among their patients and they cannot be aware of all of them.25
Medical time relating to LTFU mainly concerns: information, education, medical care, fertility, mental aspects (>80%), social aspect (63%), physical activities, and sexual aspects (<50%).
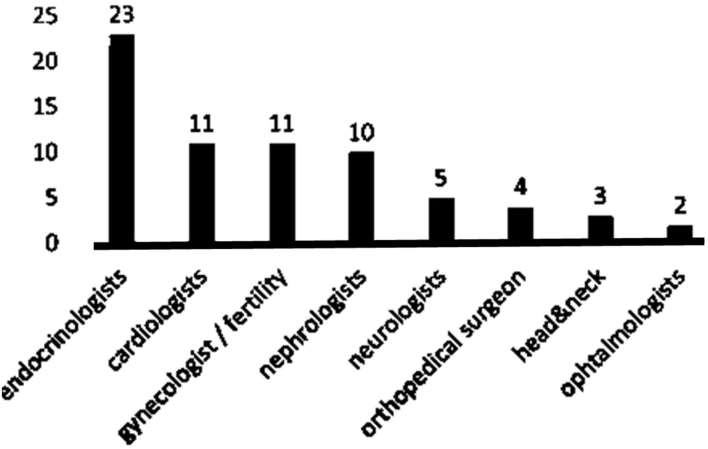
A network of specialists involved in LTFU is organized in some cities, involving mainly endocrinologists, cardiologists, gynaecologists and fertility physicians, and nephrologists (Figure 3).
Figure 3.
Network of specialists involved in long-term follow-up.
Steps relating to second cancer screenings are related to thyroid (55%), breast (34% with 26% more waiting for the national study programme, DeNaCaPST, which had not started at the time of the survey), skin (8%), colorectal (4%), and pulmonary (2%) cancer. DeNaCaPST is a non-interventional study whose aim is to assess whether international recommendations relating to second cancer screening are followed, especially with regard to breast and thyroid cancer.
Difficulties
The main problem was the lack of time to devote to LTFU (57%), despite the fact that 22% answered that some physicians in their department have time specifically dedicated for LTFU. In the same department, answers were completely different (100% discrepancy). The other difficulties were: difficulties contacting aCCSs (26%), aCCSs who ultimately do not show up to the consultation (19%), cost (15%), and lack of organization (13%). Another problem was lack of information from the GP when aCCSs attended their LTFU. Some physicians asked for specific training.
Summary—“passport”
48% of physicians reported that a summary is written for the CCS in their department. When it exists, this summary includes a cumulative dose of chemotherapy in 88% of cases, data about radiation therapy (72%), and possible fertility preservation (68%). Some departments declare that other data are systematically reported, such as use of a catheter or a prosthesis.
Ideal organization in France
Only 32/54 physicians described their vision of the ideal organization of LTFU for aCCSs in France. The answers can be summarized in 3 models.
The idea of an LTFU clinic with different levels was suggested by most physicians (72%). Two models were defined. The first model involved different specialized physicians and aCCSs would come to the clinic once a year or less depending on their needs. The second model described the clinic as a transition, starting with a consultation with the paediatric oncologist and an adult doctor or a nurse specialized in therapeutic education to help the GPs and the aCCS to coordinate the LTFU.
The role of the GP should be maintained, but with a different organization. For example, a network of the GPs involved, outside of hospitals, could be of great help, with an easy access to the specialized physicians involved (22%).
The third suggestion was to refer aCCSs to adult oncologists/haematologists (6%).
Otherwise, some physicians, mainly surgeons and radiation oncologists, find they are not sufficiently involved in LTFU and suggest, e.g. that the summary could be co-signed due to the large amount of recorded data it contains that will follow the aCCS all her/his life. The summary could include: recommendations about vaccinations, hobbies that are allowed and not allowed, and a brief summary in English.
Discussion
Whether in France or abroad, there is no single model for the follow-up of adults treated for cancer during childhood.17,26–36 In France, various organizations have already been put in place, while others are developing gradually.14, 24 The survey carried out in spring 2016 among members of the SFCE, with a satisfactory representativeness rate (70.5% of SFCE centres having responded), has highlighted a lack of communication surrounding LTFU, whose definition, objectives, and organzsation are not uniform. Lack of uniformity was also illustrated by the different answers coming from a same department [e.g. regarding potential time dedicated for LTFU and the existence of a summary (60% of discrepancy in the departments where there were several answers)]. Several documents are available on the SFCE website explaining LTFU to patients, but only 26% of physicians use these resources.14, 37,38
LTFU until the end of the paediatric age seems to be relatively uniform across the whole of France (86%) and involves the various professionals in charge of the initial treatment. As concerns the adult population, follow-up is not uniform in France and is often entrusted to the GP without further feedback. Moreover, several obstacles have been identified: the challenge and non-adherence of a varying percentage depending on the experience; the difficulties involved in finding and contacting the aCCS; the transition between the paediatric and adult worlds; and the need for paediatricians to have access to LTFU data, which may be beneficial to patients during treatment. In France, seven LTFU programmes exist and differ considerably (methods, frequency, organization, effort involved in tracking down former patients etc.).14 Our questionnaire has highlighted a modest evolution in the last 10 years. A first survey was carried out in 2006. Approximately, the same number of answers were collected (56 in 2006), however, a higher number of physicians other than paediatric oncologists answered. Thanks to a call for project in 2011, the number of LTFU programmes increased significantly, with 7 in 2016 vs 3 in 2006. The difference in the frequency of offering a summary is not significant (p = 0.15) (even with a decrease trend: 63% in 2006 and 48% in 2016). Improvements have been made in centres where these models have been implemented and the number of different professionals increased. According to the 2006 survey, LTFU care was entrusted more often to paediatricians, even when the patients were adults, and the consultations often took place in paediatric departments that were not age-appropriate. Some hospitals/centres have developed LTFU appointments that involve a range of healthcare professionals, such as a doctor and/or a specialist nurse, a joint clinic with both paediatric and adult clinicians, or specialist clinicians (e.g. a gynaecologist or reproductive health specialist). Evidence suggests that this follow-up varies depending on the working patterns and medical viewpoints of individual clinicians.
Cancer survivors are often dissatisfied with the information they receive.39 In an American online survey, more than 60% of respondents expressed a desire or need for age-appropriate information about cancer, diet, exercise, nutrition, complementary and alternative health services, infertility, mental health, and counselling.40 Until recently, there have been few services in place to resolve these problems. Since 2012, dedicated programmes have been introduced in different European cities, offering 15- to 25-year-olds mental, social, and medical support to address these specific needs. Initially, the preferred model was follow-up clinics, which were mainly run by paediatricians.26 However, the traditional hospital-based model of care is becoming increasingly unaffordable, may be not in line with the wishes of all patients, and is not clinically justified in all instances.21, 28,41,42 Another problem that has arisen is that hospital-based aftercare does not seem to be a medical model that encourages informed self-management and development of autonomy and independence.22 Greenfield et al agree with this point of view, suggesting that follow-up at the specialist main treatment centre may neither be appropriate to the needs of aCCSs nor help them to close the chapter of cancer in their lives.43
The problem of patients who do not respond to invitations to LTFU is not specific to France and ranged from 20 to 57% depending on the study,16, 18,44 despite reminders being sent to patients. These patients, who are exposed to the risk of sequelae but are not followed up medically, raise ethical considerations, ranging from loss of opportunity to unreasonable insistence on the part of physicians. It is necessary to outline each of these concepts to better adapt strategies relating to asking former patients to participate. Loss of self-confidence in the period shortly after the completion of primary treatment reveals that recovery from primary cancer treatment requires rebuilding lost confidence and that cancer survivors may struggle if their confidence is low or if they do not receive the appropriate support. Indeed, this loss of self-confidence may itself be a significant barrier to accessing support. This raises the question of the need for a short break without follow-up, depending on the different risks of sequelae and whether it is feasible and reasonable. This would allow the patient to build their identity outside of the medical world, avoiding potential fatigue with the suggested follow-up, long after the phase of active monitoring, when the risk of recurrence is significant, and before the occurrence of serious side effects. This does not concern all patients and algorithm would help clinicians determine which parts of the population to target. With such a break, the way to keep in contact with patients must be carefully planned and could be made simpler by the existence of a national remote prevention and help service. Ways to keep in touch with patients must then be considered. Several French studies are currently attempting to answer some of these questions or will soon attempt to do so.
In France, it is estimated that half of adults are not followed up by physicians who know about their health risks and are aware of LTFU recommendations. Although paediatric and radiation oncologists are the most knowledgeable healthcare providers, when it comes to late sequelae of cancer therapy in children, due to time constraints it is currently impossible for them to provide life-long monitoring, even though most radiation oncologists are adult practitioners.45 In several healthcare systems, the GP can be a good point of contact to promote follow-up care, advise patients on lifestyle choices, and carry out surveillance of late health problems and comorbidities. However, given the complexity of diagnosis and treatment-specific late side effects, patients should be provided with current and relevant information about the risks and recommendations for follow-up care and new knowledge about late side effects of cancer therapy as soon as it becomes available. Indeed, LTFU requires professionals to have specific knowledge (see national and international guidelines) and cannot be delegated without providing GPs with more information.41,46–50 The minimum they need is the summary of the illness and the treatment their patients received, and recommendations relating to LTFU care.14, 51 Despite possible co-operation with or delegation of follow-up in the future, this organization is insufficient without further feedback for the physicians in charge of cancer treatment. A systematic follow-up programme is needed to link paediatricians, oncologists, and GPs to ensure a successful transition of childhood cancer survivors from treatment and recovery to survivor care.52 Coordination is crucial because healthcare providers have a professional, ethical, and legal responsibility to inform patients, not only of the known risks associated with therapy at the time of diagnosis, but also any risks that are revealed as new information becomes available.
LTFU is a serious concern in France and a few structures have been developed to improve care and researches. The French National Registry of Childhood Cancer (RNCE) records all cancers and non-malignant intracranial tumours diagnosed in children residing in mainland France. It consists of two registries (haematological cancers, since 1990, and solid tumours, since 2000) certified for compliance with national and international requirements (INCa, Santé Publique France, INSERM). HOPE-EPI is one of the four directions of the Platform of the RNCE and records systematic follow-up of the cases diagnosed since 2000, with identification of relapses, late events, second cancers, and death (COHOPER). An additional component of HOPE-EPI is the Pedia-RT database, built by the radiation oncologists, which feeds the Platform with native dosimetry of the radiation treatments from 2013. In addition, two national cohorts focus on LTFU: the FCCSS (French Childhood Cancer Survivor Study), which is a national multicentre cohort made up of children treated for a solid tumour in France before 2000 and before the age of 21;16, 53 and LEA (Childhood and Adolescent Leukaemia), which is an open cohort initiated in 2004 with medical follow-up of the persons registered with the aim to evaluate prospectively long-term health status of childhood leukaemia survivors who were treated after 1980.24, 54 To these national cohorts can be added two regional cohorts: the Childhood Cancer Registration of the Rhône Alpes Region18 and ReCaPGO, which is a multicentre database relating to the LTFU of all children treated for cancer or a malignant haemopathy in the Grand Ouest region of France.
At this stage, remote care can be an effective tool to promote homogenous care, e.g. e-learning and virtual late effect advisory platforms.
In conclusion, LTFU is a concern for the French centres specialized in paediatric oncology of the SFCE, and involves several professionals. In France, thanks to national co-operation, we should be able to improve LTFU and promote a more homogenous access to such programmes. Irrespective of the organizational model, the main point must be the existence of a written summary and every survivor should receive such a document, after which LTFU can be started.
ACKNOWLEDGEMENTS
The authors would like to thank all the French practitioners who responded to the survey.
Contributor Information
Charlotte Demoor-Goldschmidt, Email: charlotte.demoor@inserm.fr.
Marie-Dominique Tabone, Email: marie-dominique.tabone@aphp.fr.
Valérie Bernier, Email: v.bernier@nancy.unicancer.fr.
Florent de Vathaire, Email: florent.devathaire@gustaveroussy.fr.
Claire Berger, Email: claire.berger@chu-st-etienne.fr.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin 2006; 56: 106–30. doi: 10.3322/canjclin.56.2.106 [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients. Blood 2008; 111: 2977–83. doi: 10.1182/blood-2007-10-115493 [DOI] [PubMed] [Google Scholar]
- 3.Lacour B, Goujon S, Guissou S, Guyot-Goubin A, Desmée S, Désandes E, et al. Childhood cancer survival in France, 2000–2008. Eur J Cancer Prev 2014; 23: 449–57. doi: 10.1097/CEJ.0000000000000006 [DOI] [PubMed] [Google Scholar]
- 4.Berger C, Trombert-Paviot B, Mitton N, Frappaz D, Galambrun C, Plantaz D, et al. Childhood cancer incidence and survival rates in the Rhône-Alpes regional paediatric registry 1987–1999. Arch Pediatr 2006; 13: 121–9. doi: 10.1016/j.arcped.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 5.Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, et al. Morbidity and mortality in long-term survivors of hodgkin lymphoma: a report from the childhood cancer survivor study. Blood 2011; 117: 1806–16. doi: 10.1182/blood-2010-04-278796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzi M, McMillan AJ, Siegel LS, Zumbo BD, Glickman V, Spinelli JJ, et al. Educational outcomes among survivors of childhood cancer in British Columbia, Canada: report of the childhood/adolescent/young adult cancer survivors (CAYACS) program. Cancer 2009; 115: 2234–45. doi: 10.1002/cncr.24267 [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: a report from the childhood cancer survivor study. JAMA 2003; 290: 1583–92. doi: 10.1001/jama.290.12.1583 [DOI] [PubMed] [Google Scholar]
- 8.Rueegg CS, Gianinazzi ME, Rischewski J, Beck Popovic M, von der Weid NX, Michel G, et al. Health-related quality of life in survivors of childhood cancer: the role of chronic health problems. J Cancer Surviv 2013; 7: 511–22. doi: 10.1007/s11764-013-0288-4 [DOI] [PubMed] [Google Scholar]
- 9.Chao C, Xu L, Bell E, Cooper R, Mueller L. Long-term health outcomes in survivors of childhood cancer diagnosed between 1990 and 2000 in a large US integrated health care system. J Pediatr Hematol Oncol 2016; 38: 123–30. doi: 10.1097/MPH.0000000000000492 [DOI] [PubMed] [Google Scholar]
- 10.Sadak KT, Bahr TL, Moen C, Neglia JP, Jatoi A. The clinical and research infrastructure of a childhood cancer survivor program. J Cancer Educ 2015; 30: 471–6. doi: 10.1007/s13187-014-0713-z [DOI] [PubMed] [Google Scholar]
- 11.Rebholz CE, von der Weid NX, Michel G, Niggli FK, Kuehni CE, Swiss Pediatric Oncology Group (SPOG). Follow-up care amongst long-term childhood cancer survivors: a report from the Swiss childhood cancer survivor study. Eur J Cancer 2011; 47: 221–9. doi: 10.1016/j.ejca.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 12.Eshelman-Kent D, Kinahan KE, Hobbie W, Landier W, Teal S, Friedman D, et al. Cancer survivorship practices, services, and delivery: a report from the children’s oncology group (COG) nursing discipline, adolescent/young adult, and late effects committees. J Cancer Surviv 2011; 5: 345–57. doi: 10.1007/s11764-011-0192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghim TT. Time to establish multidisciplinary childhood cancer survivorship programs in Korea. Korean J Hematol 2010; 45: 84–7. doi: 10.5045/kjh.2010.45.2.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger C, El Fayech C, Pacquement H, Demoor-Goldschmidt C, Ducassou S, Ansoborlo S, et al. Objectives and organization for the long-term follow-up after childhood cancer. Bull Cancer 2015; 102: 579–85. doi: 10.1016/j.bulcan.2015.03.022 [DOI] [PubMed] [Google Scholar]
- 15.Gianinazzi ME, Essig S, Rueegg CS, von der Weid NX, Brazzola P, Kuehni CE, et al. Information provision and information needs in adult survivors of childhood cancer. Pediatr Blood Cancer 2014; 61: 312–8. doi: 10.1002/pbc.24762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumas A, Berger C, Auquier P, Michel G, Fresneau B, Sètchéou Allodji R, et al. Educational and occupational outcomes of childhood cancer survivors 30 years after diagnosis: a French cohort study. Br J Cancer 2016; 114: 1060–8. doi: 10.1038/bjc.2016.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumas A, Cailbault I, Perrey C, Oberlin O, De Vathaire F, Amiel P. Educational trajectories after childhood cancer: when illness experience matters. Soc Sci Med 2015; 135: 67–74. doi: 10.1016/j.socscimed.2015.04.031 [DOI] [PubMed] [Google Scholar]
- 18.Bagur J, Massoubre C, Casagranda L, Faure-Conter C, Trombert-Paviot B, Berger C. Psychiatric disorders in 130 survivors of childhood cancer: preliminary results of a semi-standardized interview. Pediatr Blood Cancer 2015; 62: 847–53. doi: 10.1002/pbc.25425 [DOI] [PubMed] [Google Scholar]
- 19.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol 2006; 24: 5117–24. doi: 10.1200/JCO.2006.07.0474 [DOI] [PubMed] [Google Scholar]
- 20.Cox A, Faithfull S. ‘They’re survivors physically but we want them to survive mentally as well’: health care professionals’ views on providing potential late effect information. Support Care Cancer 2013; 21: 2491–7. doi: 10.1007/s00520-013-1806-7 [DOI] [PubMed] [Google Scholar]
- 21.Wallace WH, Blacklay A, Eiser C, Davies H, Hawkins M, Levitt GA, et al. Developing strategies for long term follow up of survivors of childhood cancer. BMJ 2001; 323: 271–4. doi: 10.1136/bmj.323.7307.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaser A, Levitt G, Morris P, Tapp J, Gibson F, Children and Young People workstream of the National Cancer Survivor Initiative (NCSI), UK. Enhanced quality and productivity of long-term aftercare of cancer in young people. Arch Dis Child 2013; 98: 818–24. doi: 10.1136/archdischild-2013-304348 [DOI] [PubMed] [Google Scholar]
- 23.Ross CJ, Katzov-Eckert H, Dubé MP, Brooks B, Rassekh SR, Barhdadi A, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet 2009; 41: 1345–9. doi: 10.1038/ng.478 [DOI] [PubMed] [Google Scholar]
- 24.Berbis J, Michel G, Baruchel A, Bertrand Y, Chastagner P, Demeocq F, et al. Cohort profile: the French childhood cancer survivor study for leukaemia (LEA Cohort). Int J Epidemiol 2015; 44: 49–57. doi: 10.1093/ije/dyu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger C, Casagranda L, Faure-Conter C, Freycon C, Isfan F, Robles A, et al. Long-term follow-up consultation after childhood cancer in the Rhône-Alpes region of France: feedback from adult survivors and their general practitioners. J Adolesc Young Adult Oncol 2017; 6: 524–34. doi: 10.1089/jayao.2017.0019 [DOI] [PubMed] [Google Scholar]
- 26.Oeffinger KC, Eshelman DA, Tomlinson GE, Buchanan GR. Programs for adult survivors of childhood cancer. J Clin Oncol 1998; 16: 2864–7. doi: 10.1200/JCO.1998.16.8.2864 [DOI] [PubMed] [Google Scholar]
- 27.Ganju RG, Nanda RH, Esiashvili N, Switchenko JM, Wasilewski-Masker K, Marchak JG. The effect of transition clinics on knowledge of diagnosis and perception of risk in young adult survivors of childhood cancer. J Pediatr Hematol Oncol 2016; 38: 197–201. doi: 10.1097/MPH.0000000000000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox CL, Zhu L, Ojha RP, Li C, Srivastava DK, Riley BB, et al. The unmet emotional, care/support, and informational needs of adult survivors of pediatric malignancies. J Cancer Surviv 2016; 10: 743–58. doi: 10.1007/s11764-016-0520-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winther JF, Kenborg L, Byrne J, Hjorth L, Kaatsch P, Kremer LC, et al. Childhood cancer survivor cohorts in Europe. Acta Oncol 2015; 54: 655–68. doi: 10.3109/0284186X.2015.1008648 [DOI] [PubMed] [Google Scholar]
- 30.Hjorth L, Haupt R, Skinner R, Grabow D, Byrne J, Karner S, et al. Survivorship after childhood cancer: PanCare: a European network to promote optimal long-term care. Eur J Cancer 2015; 51: 1203–11. doi: 10.1016/j.ejca.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salz T, McCabe MS, Onstad EE, Baxi SS, Deming RL, Franco RA, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer 2014; 120: 722–30. doi: 10.1002/cncr.28472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oeffinger KC, Argenbright KE, Levitt GA, McCabe MS, Anderson PR, Berry E, et al. Models of cancer survivorship health care: moving forward. Am Soc Clin Oncol Educ Book 2014;: 205–13. doi: https://doi.org/10.14694/EdBook_AM.2014.34.205 [DOI] [PubMed] [Google Scholar]
- 33.Hashmi S, Carpenter P, Khera N, Tichelli A, Savani BN. Lost in transition: the essential need for long-term follow-up clinic for blood and marrow transplantation survivors. Biol Blood Marrow Transplant 2015; 21: 225–32. doi: 10.1016/j.bbmt.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 34.Skinner R, Oeffinger KC. Developing international consensus for late effects screening and guidance. Curr Opin Support Palliat Care 2013; 7: 1–8. doi: 10.1097/SPC.0b013e328363a607 [DOI] [PubMed] [Google Scholar]
- 35.Sadak KT, Connor C, DeLuca H. Innovative educational approaches to engage and empower the adolescent and young adult childhood cancer survivor. Pediatr Blood Cancer 2013; 60: 1919–21. doi: 10.1002/pbc.24635 [DOI] [PubMed] [Google Scholar]
- 36.Jereb B. Model for long-term follow-up of survivors of childhood cancer. Med Pediatr Oncol 2000; 34: 256–8. doi: [DOI] [PubMed] [Google Scholar]
- 37.Demoor-Goldschmidt C, Bernier V. Towards an improvement of the quality of life after radiotherapy in children. Bull Cancer 2015; 102: 674–83. doi: 10.1016/j.bulcan.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 38.Do Nascimento C, Demoor-Goldschmidt C. SALTO: édition et mise à disposition d’une nouvelle bande dessinée adressée aux patients permettant d’expliquer les intérêts d’une consultation de suivi à long terme. Revue d'Oncologie Hématologie Pédiatrique 2016; 4: 74–5. doi: 10.1016/j.oncohp.2015.12.001 [DOI] [Google Scholar]
- 39.Ross L, Petersen MA, Johnsen AT, Lundstrøm LH, Groenvold M. Satisfaction with information provided to Danish cancer patients: validation and survey results. Patient Educ Couns 2013; 93: 239–47. doi: 10.1016/j.pec.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 40.Zebrack B. Information and service needs for young adult cancer survivors. Support Care Cancer 2009; 17: 349–57. doi: 10.1007/s00520-008-0469-2 [DOI] [PubMed] [Google Scholar]
- 41.Blaauwbroek R, Barf HA, Groenier KH, Kremer LC, van der Meer K, Tissing WJ, et al. Family doctor-driven follow-up for adult childhood cancer survivors supported by a web-based survivor care plan. J Cancer Surviv 2012; 6: 163–71. doi: 10.1007/s11764-011-0207-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson F, Soanes L. Long-term follow-up following childhood cancer: maximising the contribution from nursing. Eur J Cancer 2001; 37: 1859–66. [DOI] [PubMed] [Google Scholar]
- 43.Greenfield DM, Absolom K, Eiser C, Walters SJ, Michel G, Hancock BW, et al. Follow-up care for cancer survivors: the views of clinicians. Br J Cancer 2009; 101: 568–74. doi: 10.1038/sj.bjc.6605160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009; 27: 2308–18. doi: 10.1200/JCO.2009.22.3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demoor-Goldschmidt C, Claude L, Carrie C, Bolle S, Helfre S, Alapetite C, et al. French organization of paediatric radiation treatment: Results of a survey conducted by the radiotherapy committee of the French society of paediatric cancers (SFCE). Cancer Radiother 2016; 20: 395–9. doi: 10.1016/j.canrad.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 46.Volerman A. Primary care of the childhood cancer survivor. Med Clin North Am 2015; 99: 1059–73. doi: 10.1016/j.mcna.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 47.Suh E, Daugherty CK, Wroblewski K, Lee H, Kigin ML, Rasinski KA, et al. General internists' preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med 2014; 160: 11-17–17. doi: 10.7326/M13-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nathan PC, Daugherty CK, Wroblewski KE, Kigin ML, Stewart TV, Hlubocky FJ, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv 2013; 7: 275–82. doi: 10.1007/s11764-013-0271-0 [DOI] [PubMed] [Google Scholar]
- 49.McCabe MS, Partridge AH, Grunfeld E, Hudson MM. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Semin Oncol 2013; 40: 804–12. doi: 10.1053/j.seminoncol.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kremer LC, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the international late effects of childhood cancer guideline harmonization group. Pediatr Blood Cancer 2013; 60: 543–9. doi: 10.1002/pbc.24445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horowitz ME, Fordis M, Krause S, McKellar J, Poplack DG. Passport for care: implementing the survivorship care plan. J Oncol Pract 2009; 5: 110–2. doi: 10.1200/JOP.0934405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aziz NM, Oeffinger KC, Brooks S, Turoff AJ. Comprehensive long-term follow-up programs for pediatric cancer survivors. Cancer 2006; 107: 841–8. doi: 10.1002/cncr.22096 [DOI] [PubMed] [Google Scholar]
- 53.Haddy N, El-Fayech C, Guibout C, Adjadj E, Thomas-Teinturier C, Oberlin O, et al. Thyroid adenomas after solid cancer in childhood. Int J Radiat Oncol Biol Phys 2012; 84: e209–e215. doi: 10.1016/j.ijrobp.2012.03.044 [DOI] [PubMed] [Google Scholar]
- 54.Sirvent A, Auquier P, Oudin C, Bertrand Y, Bohrer S, Chastagner P, et al. Prevalence and risk factors of iron overload after hematopoietic stem cell transplantation for childhood acute leukemia: a LEA study. Bone Marrow Transplant 2017; 52: 80–7. doi: 10.1038/bmt.2016.205 [DOI] [PubMed] [Google Scholar]